The Iodine Clock Reaction

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
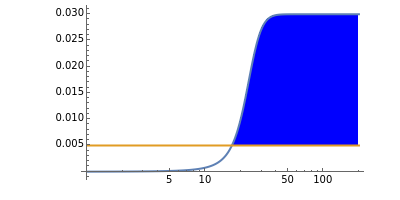
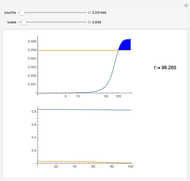
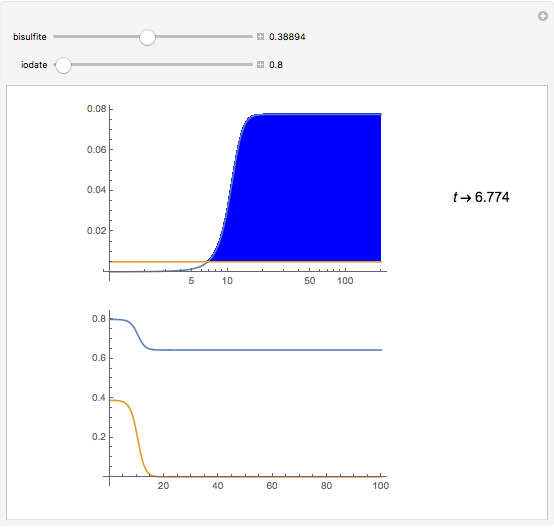
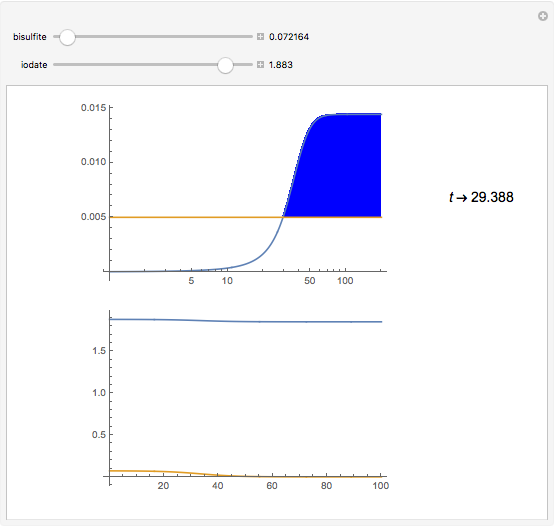
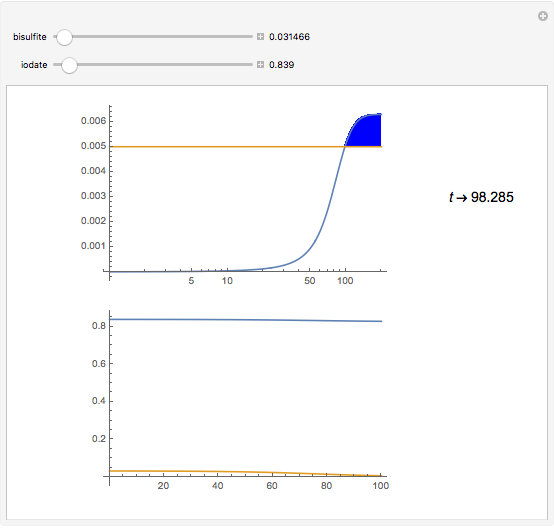
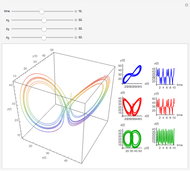
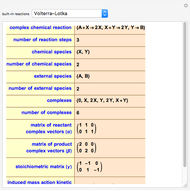
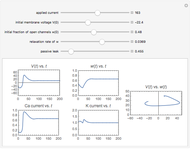
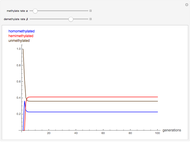
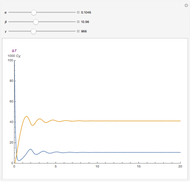
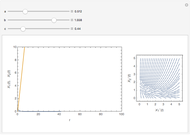
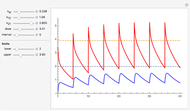
The iodine clock reaction (also known as the Harcourt–Esson reaction) is a classic chemical clock experiment for displaying chemical kinetics in action; it was discovered by Hans Heinrich Landolt in 1886. Two colorless solutions are mixed and at first there is no visible reaction. After a short, predictable time delay, the liquid suddenly turns a shade of dark blue.
[more]
Contributed by: Benson R. Sundheim (December 2011)
Open content licensed under CC BY-NC-SA
Snapshots
Details
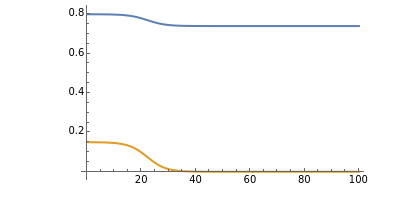
The iodide ion is generated by the slow reaction between the iodate and bisulfite:
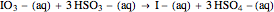 .
.
The iodate also oxidizes the generated iodide to form iodine:
 .
.
The iodine is reduced immediately back to iodide by the bisulfite:
 .
.
When the bisulfite is fully consumed, the iodine will accumulate (i.e., not be reduced by the bisulfite) and form the dark blue complex with starch.
Permanent Citation