Half-Life of a Radioactive Decay

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
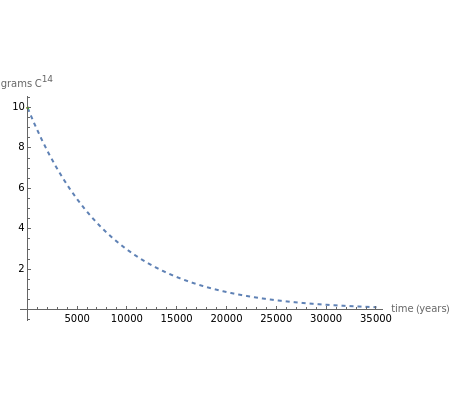
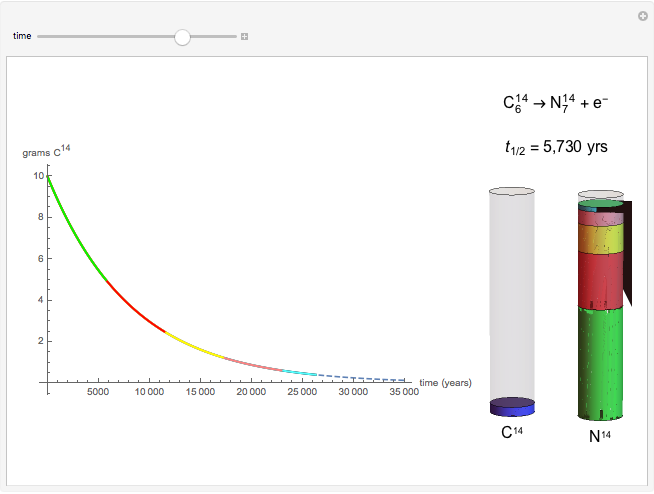
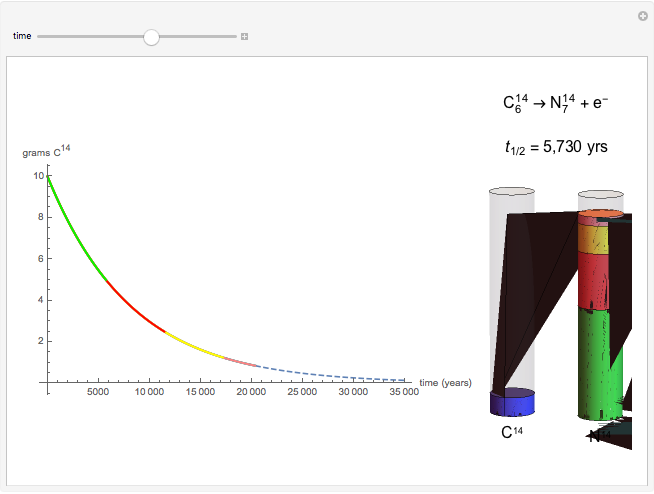
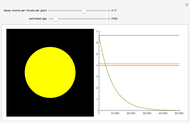
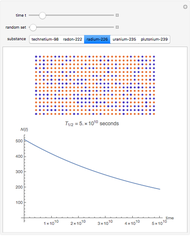
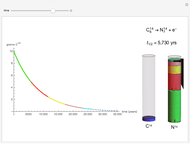
The half-life of a reaction is the time required for the amount of reactant to decrease by a half. The half-life of a first-order reaction is a constant, so each successive half-life represents an equal period of time. For example, carbon-14 decays to nitrogen-14 by the emission of an electron with a half-life of 5,730 years. This Demonstration illustrates how an initial sample of 10 grams of carbon-14 decays to nitrogen-14 over 34,380 years.
Contributed by: Kyle Tomczak, John Ziebec, Stephen Koveck, Hiral Bharatia, and Chris Sicinski (December 2013)
Indiana University Northwest Department of Chemistry, Physics, and Astronomy
Additional Contributions by: Axel Schulze-Halberg, Daniel Kelly, and Nelson DeLeon
Open content licensed under CC BY-NC-SA
Snapshots
Details
detailSectionParagraphPermanent Citation
"Half-Life of a Radioactive Decay"
http://demonstrations.wolfram.com/HalfLifeOfARadioactiveDecay/
Wolfram Demonstrations Project
Published: December 20 2013