Hydrogen Fuel Cell

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
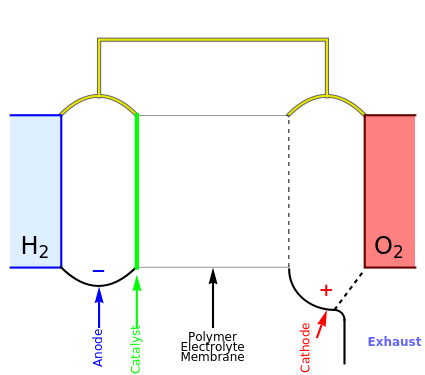
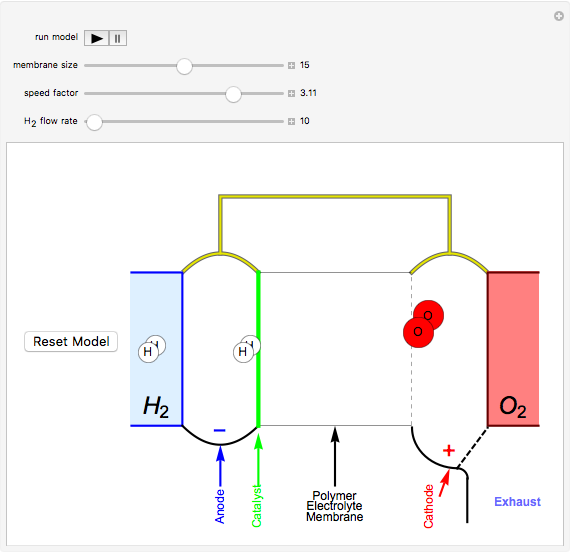
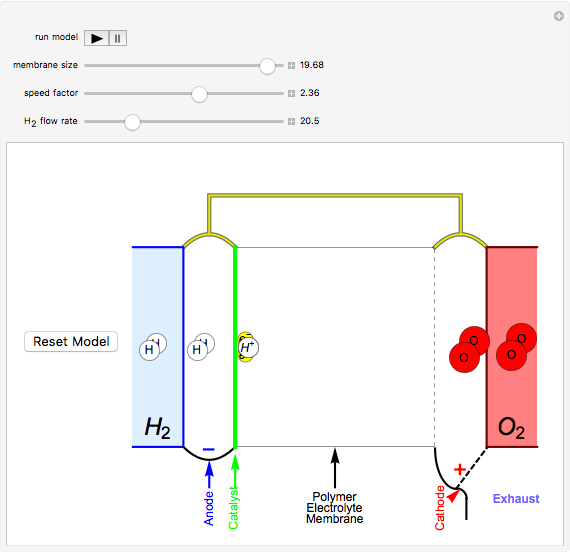
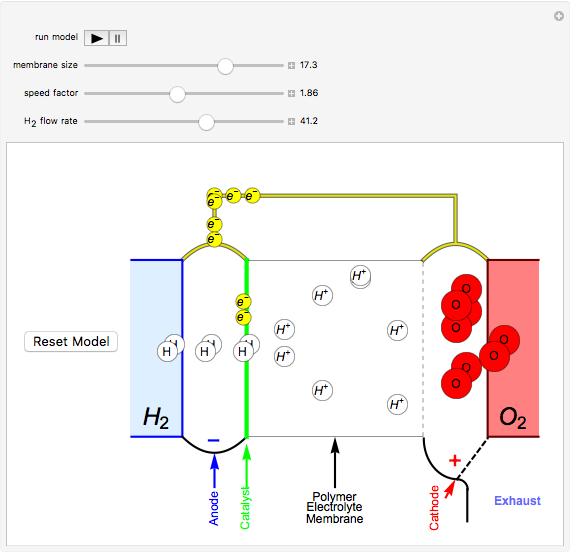
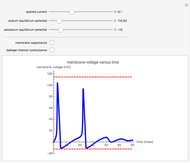
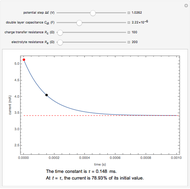
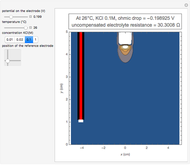
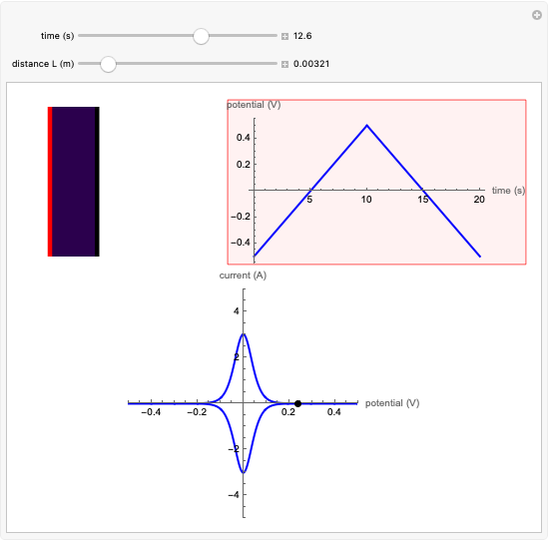
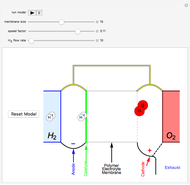
This Demonstration shows a simple model of a hydrogen fuel cell. Hydrogen molecules flow from the blue tank through the catalyst where electrons are removed, and proceed toward the cathode. The electrons form a current through the external wire, ending up in the cathode and recombining with the hydrogen ions to regenerate hydrogen gas. Oxygen gas flows from the red tank and hydrogen peroxide is produced in the reaction with hydrogen gas. This in turn dissociates into water plus another hydrogen molecule. The water then passes through the external exhaust pipe as "waste".
Contributed by: Luo Qian (May 2012)
Open content licensed under CC BY-NC-SA
Snapshots
Details
Permanent Citation
"Hydrogen Fuel Cell"
http://demonstrations.wolfram.com/HydrogenFuelCell/
Wolfram Demonstrations Project
Published: May 29 2012