A Second-Order Chemical Reaction
Initializing live version

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
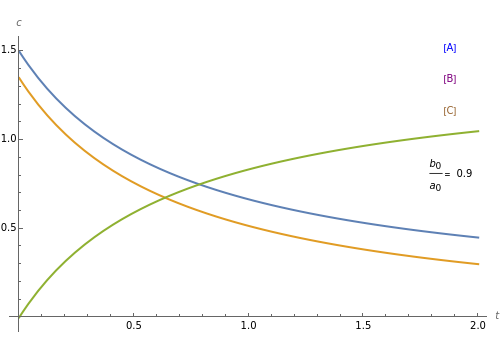
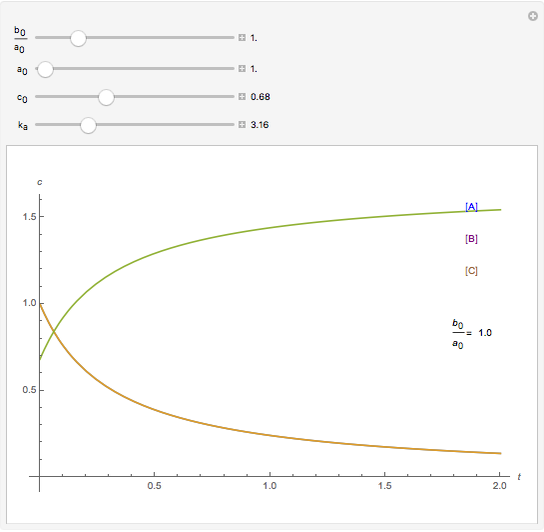
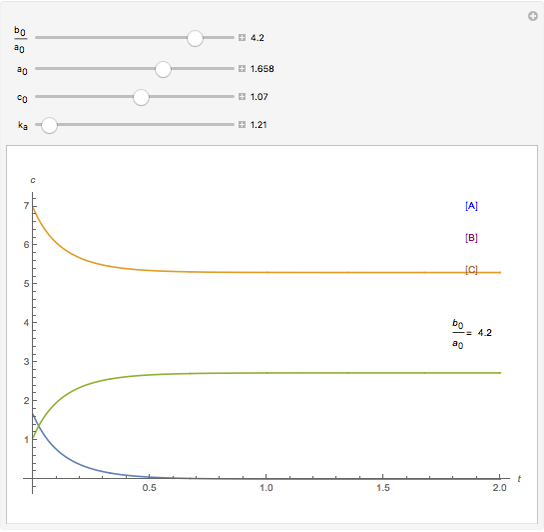
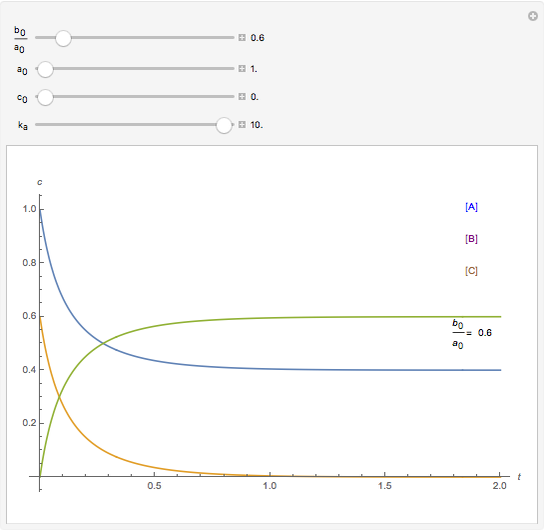
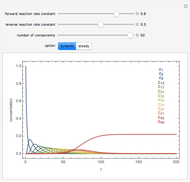
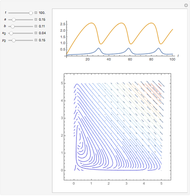
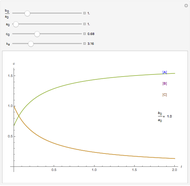
A reaction of second order A + B → C is shown with initial concentrations  ,
,  , and
, and  . The rate constant
. The rate constant  and the initial conditions are variable. Displayed are the time dependent concentrations that are solutions to the corresponding differential equations:
and the initial conditions are variable. Displayed are the time dependent concentrations that are solutions to the corresponding differential equations:
Contributed by: Gergard Schwaab and Chantal Lorbeer (Ruhr University Bochum) (March 2011)
Open content licensed under CC BY-NC-SA
Snapshots
Details
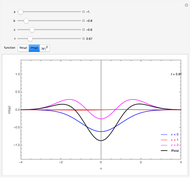
Solutions of the differential equations:



For the special case,  :
:



Permanent Citation