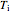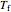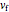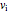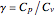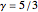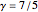Adiabatic Expansion and Compression of an Ideal Gas

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
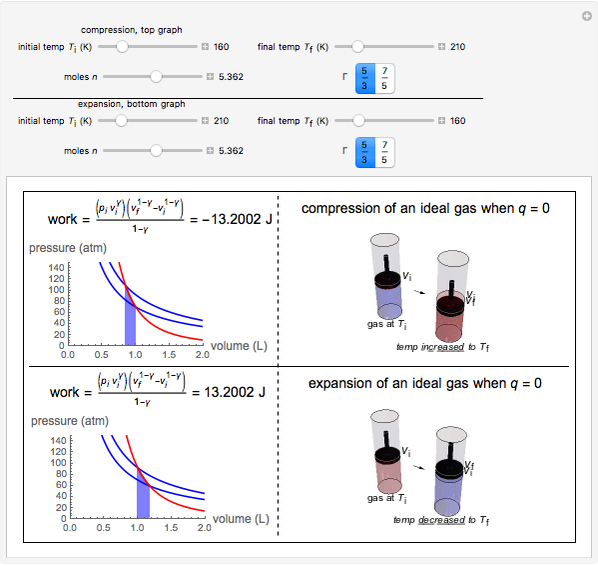
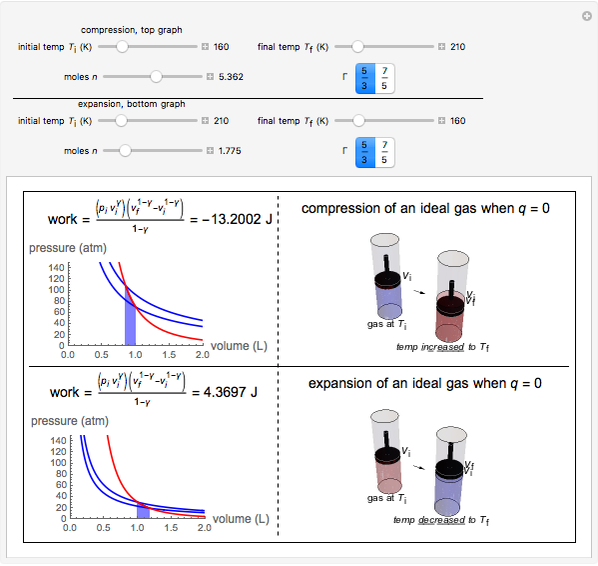
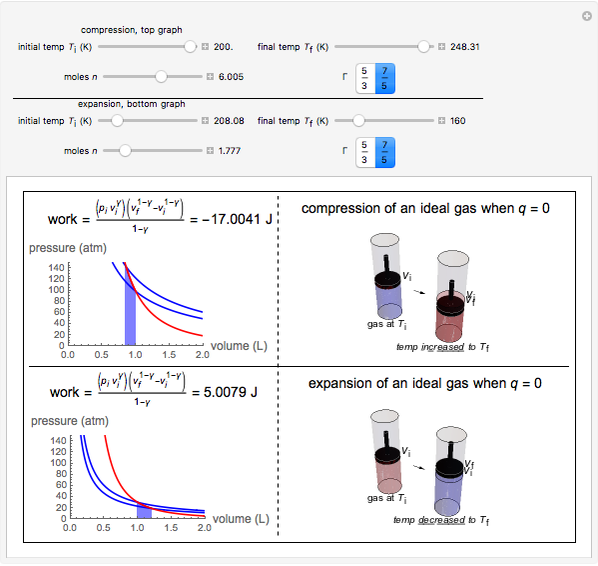
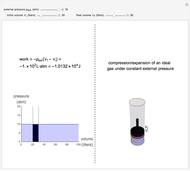
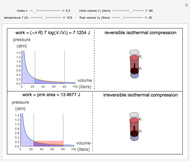
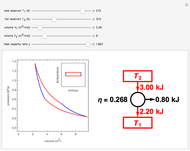
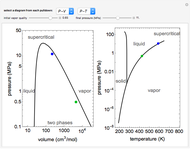
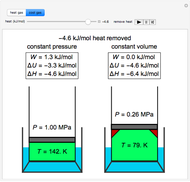
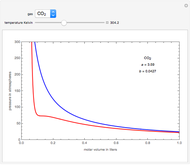
This Demonstration shows adiabatic compression/expansions of an ideal gas, a frequently invoked thermodynamic process (e.g., in a Carnot cycle). Each graphic displays three curves. The two blue curves represent the isotherms of the initial and final temperature; the red line is the adiabatic curve.
[more]
Contributed by: Blair Winograd (June 2015)
Open content licensed under CC BY-NC-SA
Snapshots
Details
Reference
[1] P. W. Atkins and L. Jones, Chemical Principles: The Quest for Insight, New York: W. H. Freeman, 1999.
Permanent Citation
"Adiabatic Expansion and Compression of an Ideal Gas"
http://demonstrations.wolfram.com/AdiabaticExpansionAndCompressionOfAnIdealGas/
Wolfram Demonstrations Project
Published: June 10 2015