Beer's Law

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
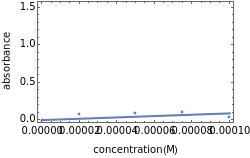
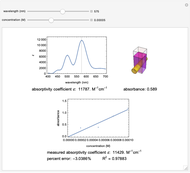
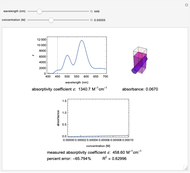
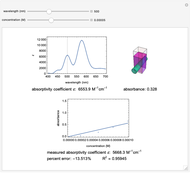
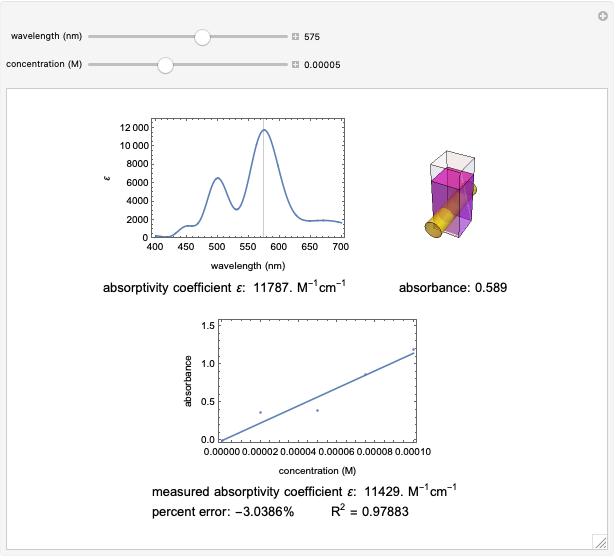
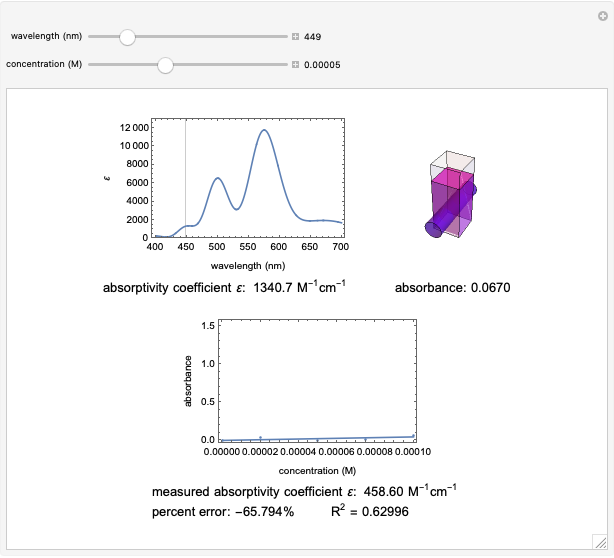
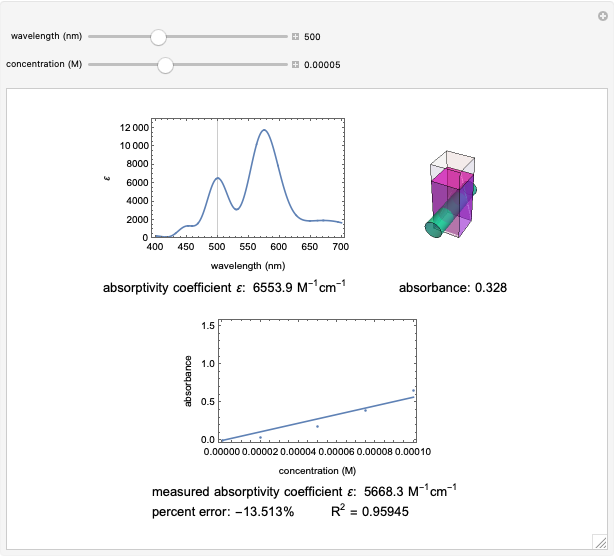
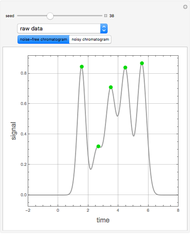
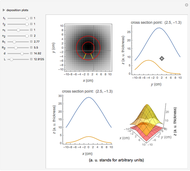
Spectroscopy, the study of the interaction of matter with electromagnetic radiation, can be used for qualitative and quantitative analysis. This Demonstration shows the importance of choosing the appropriate wavelength at which to analyze a hypothetical sample solution containing an analyte in a noninteracting solvent. Additionally, the effects of solution concentration can be explored using Beer’s law:  , where
, where 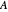 is absorbance, ϵ is the molar absorbtivity (in L
is absorbance, ϵ is the molar absorbtivity (in L 
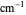 ),
),  is the path length of the sample (in cm) and
is the path length of the sample (in cm) and  is the concentration of the compound in solution (in mol
is the concentration of the compound in solution (in mol 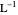 ).
).
Contributed by: Scott Berger, Rachael Holappa and Kaitlin Nguyen (May 2019)
Project was overseen by Prof. Heidi Hendrickson at Lafayette College, Easton PA.
Open content licensed under CC BY-NC-SA
Snapshots
Details
The spectrum provided is generated using [1].
Reference
[1] "Creating UV/Visible Plots from the Results of Excited States Calculations." (May 22, 2019) gaussian.com/uvvisplot.
Permanent Citation