Charles's Law
Initializing live version

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
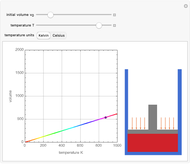
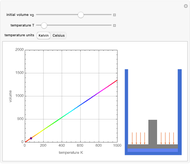
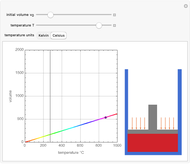
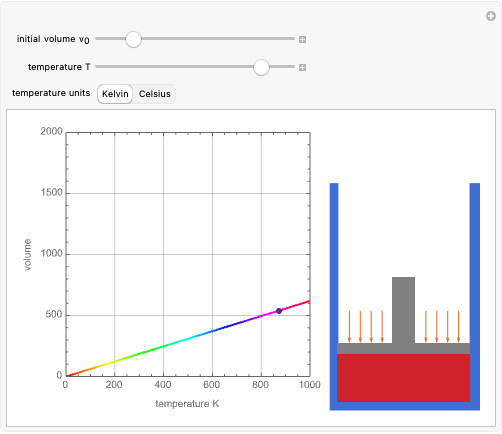
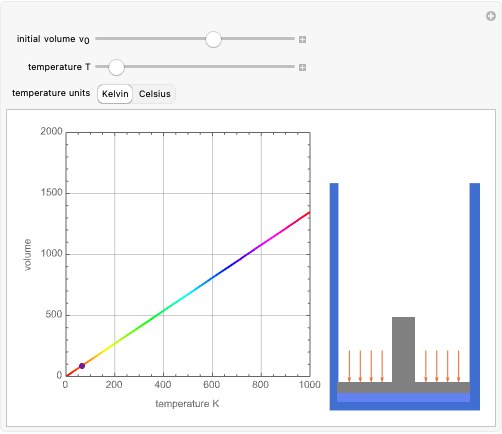
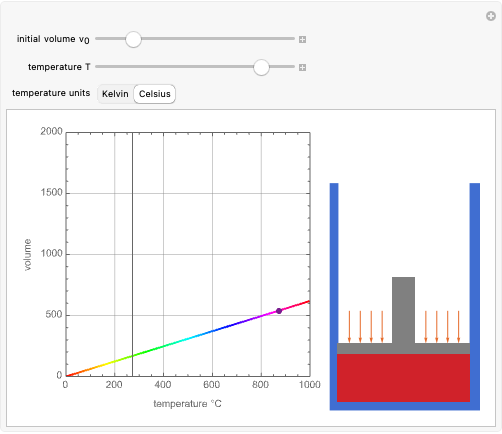
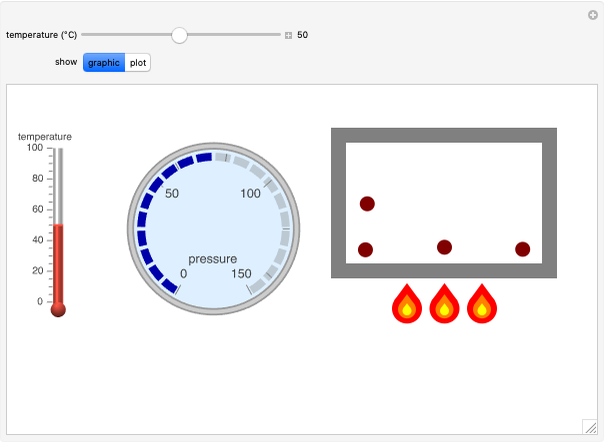
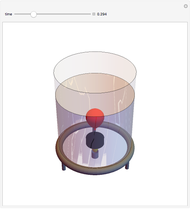
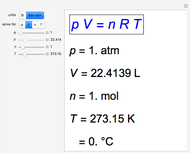
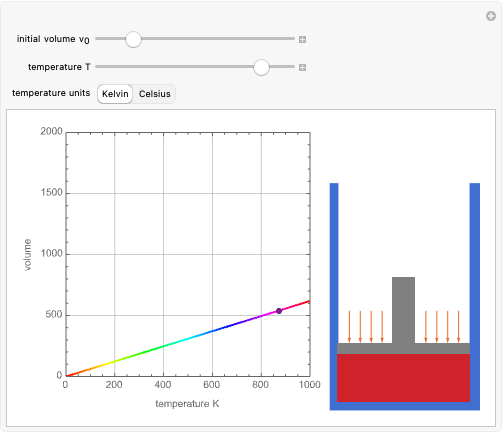
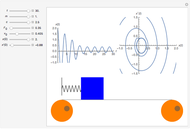
Charles's law says that the volume 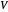 of a gas when pressure is constant is directly proportional to the absolute temperature
of a gas when pressure is constant is directly proportional to the absolute temperature 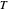 . The plot shows this proportionality for a fixed initial volume. The variation of the temperature and the volume is shown as a point in the plot and in the piston on the right side. The generalization of this law is embodied in the ideal gas law.
. The plot shows this proportionality for a fixed initial volume. The variation of the temperature and the volume is shown as a point in the plot and in the piston on the right side. The generalization of this law is embodied in the ideal gas law.
Contributed by: Enrique Zeleny (September 2008)
Open content licensed under CC BY-NC-SA
Details
Snapshots
Permanent Citation