Coordination in Ionic Compounds

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
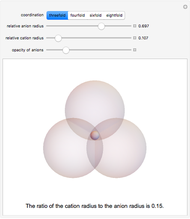
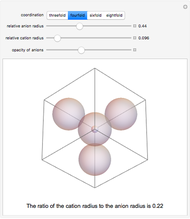
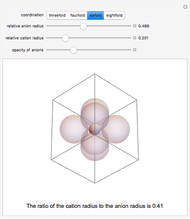
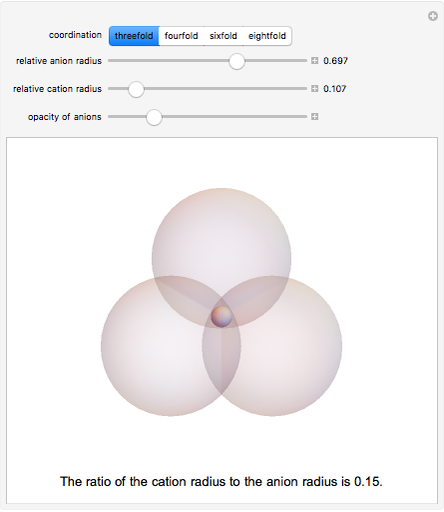
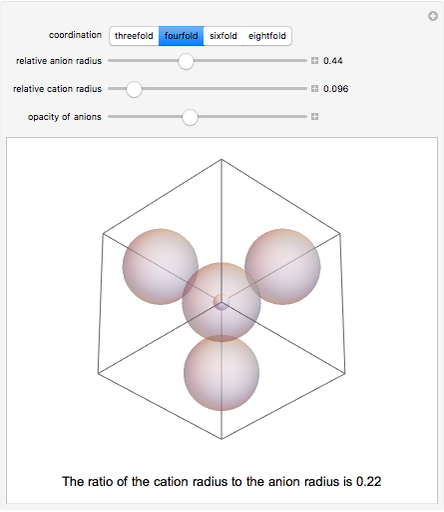
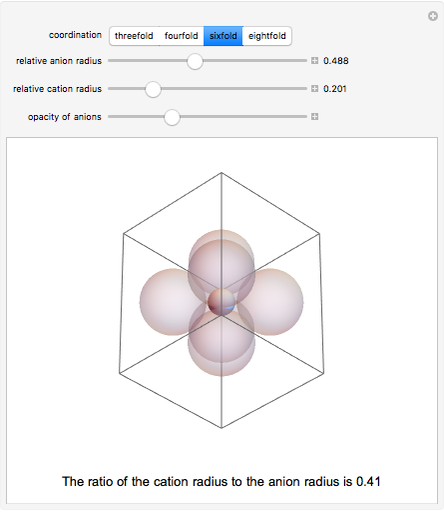
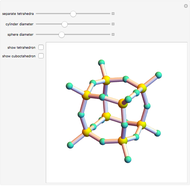
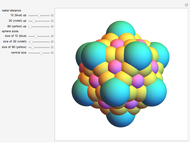
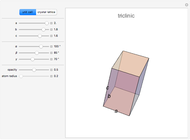
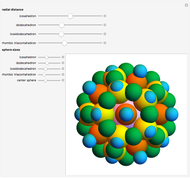
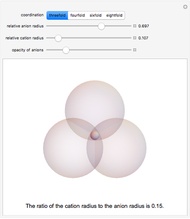
Depending on the radii of the anions and cations, some ionic compounds have threefold, fourfold, sixfold, or eightfold coordination about the cation (three nearest-neighbor anions). This Demonstration allows the user to explore the ratio of the cation to anion radii in order to determine the ideal ratio. Because of electrostatic considerations, the minimum (and ideal) ratio occurs when the anions just make contact with one another and the cation just makes contact with each anion.
Contributed by: Megan Frary (Boise State University) (March 2011)
Open content licensed under CC BY-NC-SA
Snapshots
Details
Snapshot 1: For threefold coordination, the minimum stable ratio is 0.15.
Snapshot 2: For fourfold coordination, the minimum stable ratio is 0.22.
Snapshot 3: For sixfold coordination, the minimum stable ratio is 0.41.
Snapshot 4: For eightfold coordination, the minimum stable ratio is 0.73.
Permanent Citation
"Coordination in Ionic Compounds"
http://demonstrations.wolfram.com/CoordinationInIonicCompounds/
Wolfram Demonstrations Project
Published: March 7 2011