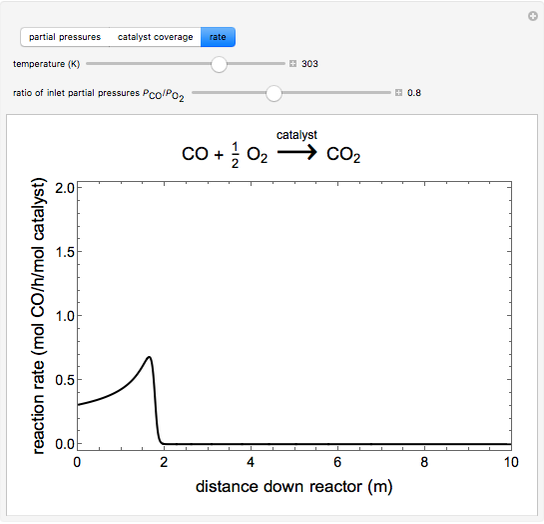
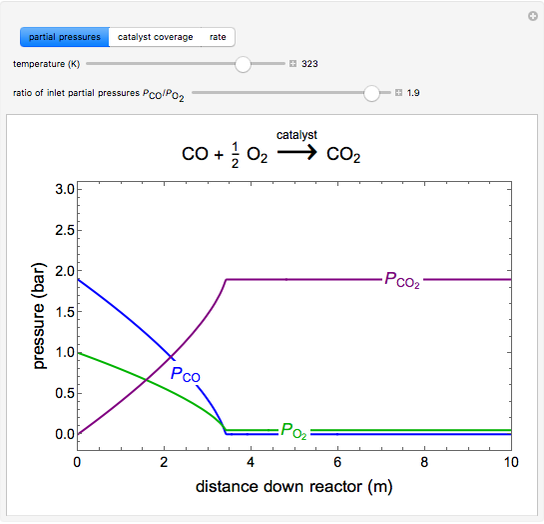
Langmuir-Hinshelwood Reaction in a Plug Flow Reactor

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
Oxygen oxidizes 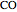 to
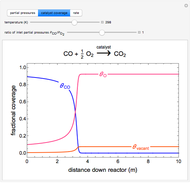
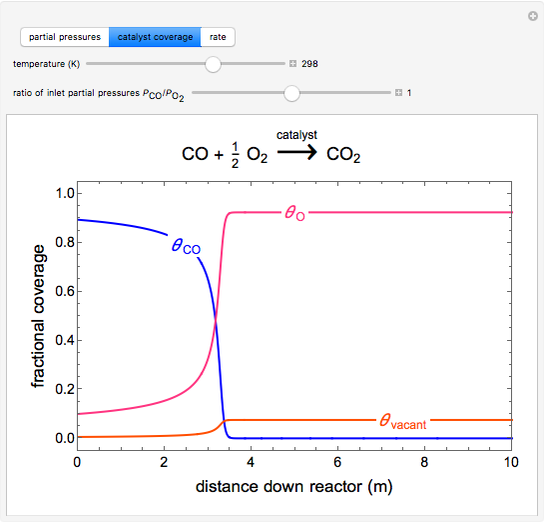
to 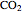 on a supported metal catalyst in an isothermal plug flow reactor. Use buttons to select plots of partial pressures, catalyst coverage or reaction rate as a function of distance down the reactor. Use sliders to vary the temperature and the ratio of the
on a supported metal catalyst in an isothermal plug flow reactor. Use buttons to select plots of partial pressures, catalyst coverage or reaction rate as a function of distance down the reactor. Use sliders to vary the temperature and the ratio of the 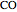 feed partial pressure to the
feed partial pressure to the 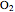 feed partial pressure. Because the rate-determining step is the reaction of adsorbed
feed partial pressure. Because the rate-determining step is the reaction of adsorbed 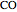 and adsorbed
and adsorbed 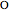 , and because
, and because 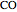 adsorbs more strongly than
adsorbs more strongly than 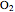 , increasing the
, increasing the 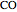 feed partial pressure can decrease the rate of
feed partial pressure can decrease the rate of 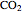 formation. This happens when the
formation. This happens when the 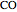 surface coverage becomes much higher than the
surface coverage becomes much higher than the 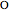 surface coverage.
surface coverage.
Contributed by: Garrison J. Vigil (December 2015)
Additional contributions by: Rachael L. Baumann, John L. Falconer, J. Will Medlin, and Nick Bongiardina
(University of Colorado Boulder, Department of Chemical and Biological Engineering)
Open content licensed under CC BY-NC-SA
Snapshots
Details
Assumptions:
1. the rate-determining step is the surface reaction between carbon monoxide 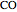 and atomic oxygen
and atomic oxygen 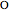
2. carbon dioxide 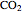 rapidly desorbs from catalyst surface
rapidly desorbs from catalyst surface
3. ideal plug flow reactor.
The fractional coverages 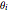 for the reaction
for the reaction 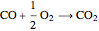 are determined from Langmuir isotherms:
are determined from Langmuir isotherms:
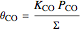 ,
,
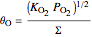 ,
,
 ,
,
for simplicity, 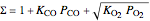 ,
,
where 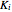 is the adsorption equilibrium constant of species
is the adsorption equilibrium constant of species 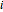 and
and 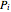 is the partial pressure.
is the partial pressure.
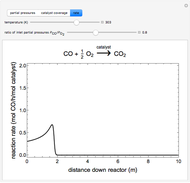
The rate of reaction  is:
is:
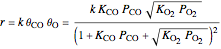 ,
,
where  is the rate constant.
is the rate constant.
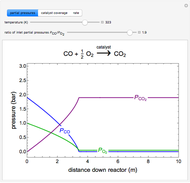
The partial pressures of 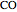 ,
, 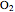 and
and 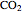 are calculated from material balances:
are calculated from material balances:
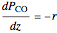 ,
,
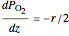 ,
,
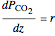 ,
,
where  is distance down the reactor.
is distance down the reactor.
Reference
[1] Langmuir Isotherms for a Binary Mixture [Video]. (Sep 1, 2016) www.colorado.edu/learncheme/kinetics/LanmuirIsothermsBinaryMixture.html.
Permanent Citation