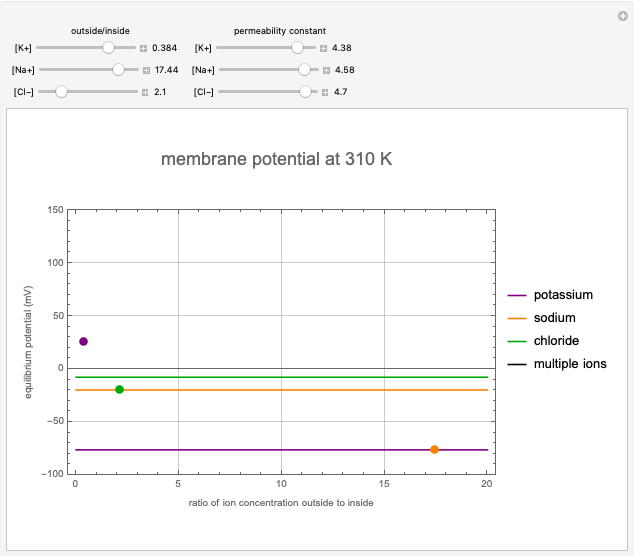
Membrane Potential Responses to Ion Gradients

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
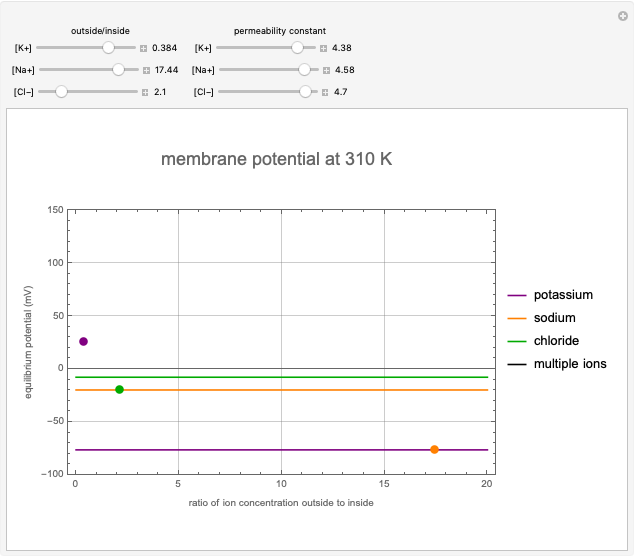
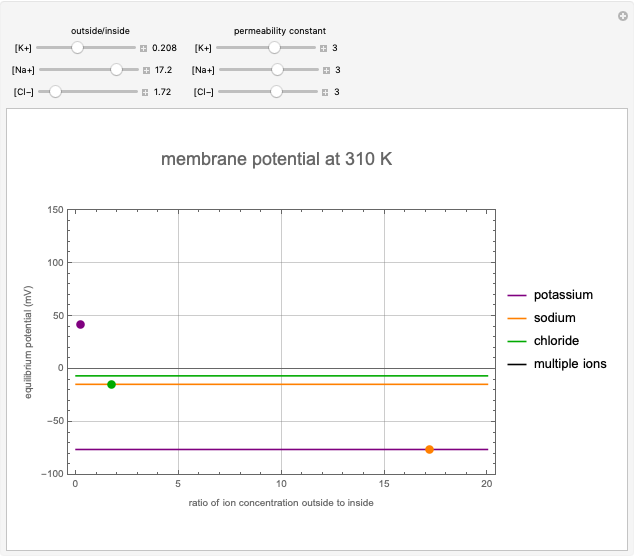
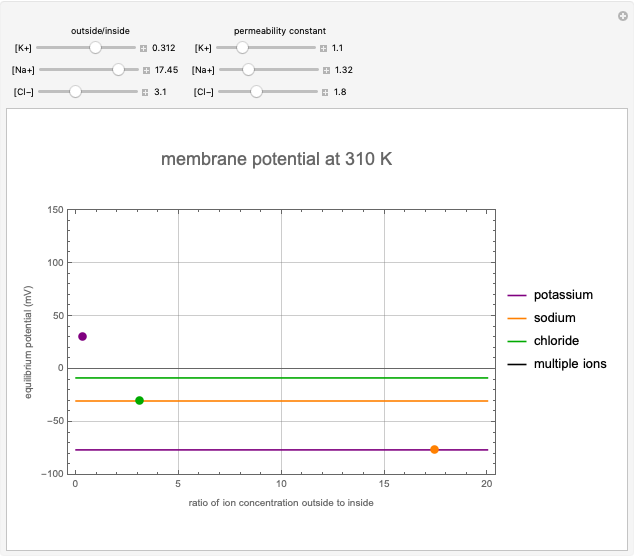
This Demonstration shows the effects of ion concentration gradients across a membrane. We also consider the membrane permittivity for an ion acted upon by the membrane potential in a generic cell, as described by the Goldman–Hodgkin–Katz equation. Variation of the concentration of an ion (inside or outside the cell) with a higher membrane permittivity constant will mostly affect the membrane potential. For a membrane that is permeable to one ion only, permittivity constants in the numerator and denominator of the Goldman equation will cancel and the potential will depend only on the ratio of ion concentrations on the two sides of the membrane. In such instances, the Goldman equation reduces to the Nernst equation.
Contributed by: Sreya Gogineni, Nitiyaja Kumar and Suhayb Ali (August 2022)
Additional contributions by: Dalia M. Hassan, Yifan Lai and Kristina M. Lenn
Open content licensed under CC BY-NC-SA
Snapshots
Details
References
[1] E. Niebur. "Electrical Properties of Cell Membranes." Scholarpedia, 3(6), 2008 7166. doi:10.4249/scholarpedia.7166.
[2] A. L. Hodgkin, A. F. Huxley and B. Katz, "Measurement of Current-Voltage Relations in the Membrane of the Giant Axon of Loligo," The Journal of Physiology, 116(4), 1952 pp. 424–448. doi:10.1113%2Fjphysiol.1952.sp004716.
[3] D. Purves, G. J. Augustine, D. Fitzpatrick, et al. (eds.), "The Forces That Create Membrane Potentials," Neuroscience, 2nd ed., Sunderland, MA: Sinauer Associates, 2001. www.ncbi.nlm.nih.gov/books/NBK11102.
[4] H. Tamagawa, "Mathematical Expression of Membrane Potential Based on Ling's Adsorption Theory Is Approximately the Same as the Goldman–Hodgkin–Katz Equation," Journal of Biological Physics, 45(1), 2019 pp. 13–30. doi:10.1007/s10867-018-9512-9.
Permanent Citation