CO2 Sublimation and Dissolution in Water

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
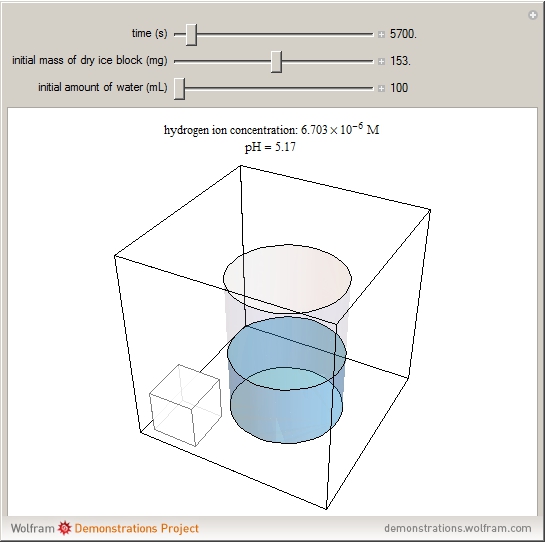
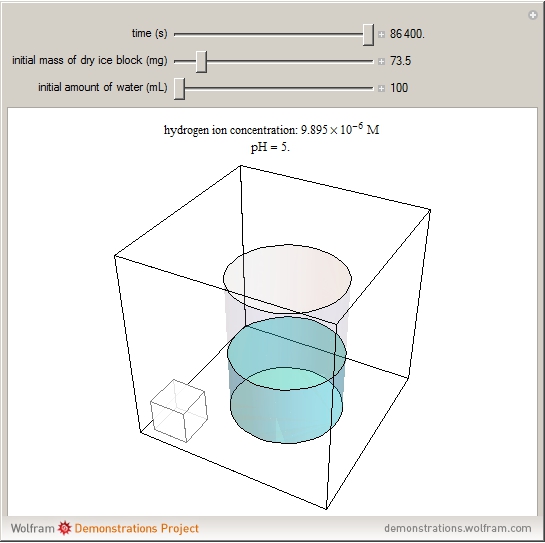
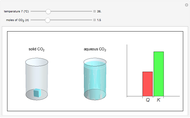
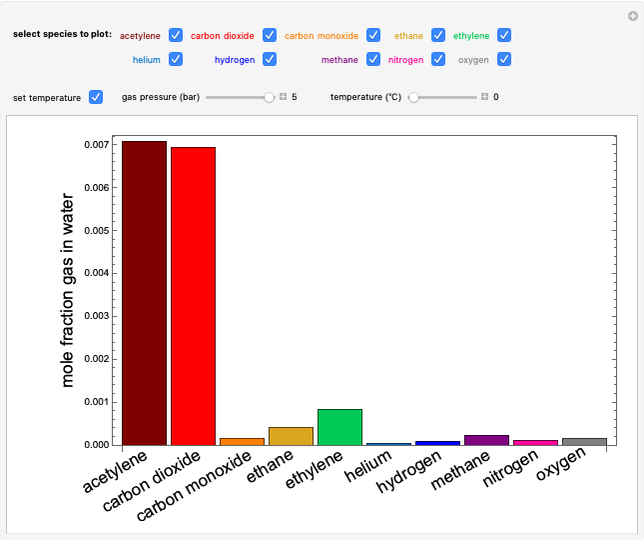
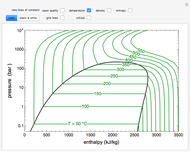
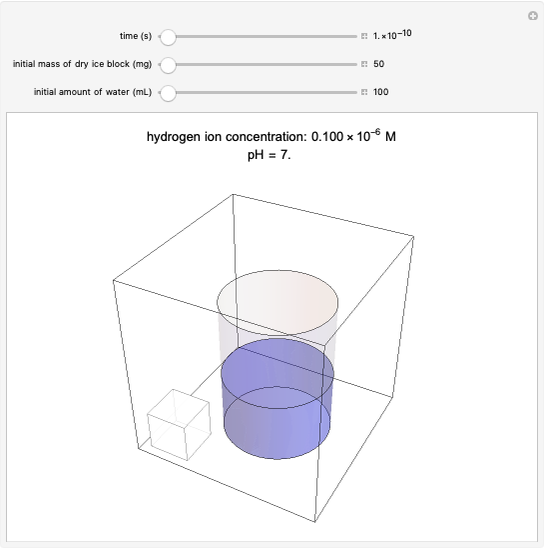
 sublimation from dry ice is a well-known phenomenon, which occurs at standard temperature and pressure. If water is accessible to the gaseous
sublimation from dry ice is a well-known phenomenon, which occurs at standard temperature and pressure. If water is accessible to the gaseous  , it will dissolve in the water. Carbonic acid and bicarbonate ions form in the sequence of chemical equilibria:
, it will dissolve in the water. Carbonic acid and bicarbonate ions form in the sequence of chemical equilibria:  Dissociation of the carbonic acid causes the pH of the water to decrease.
Dissociation of the carbonic acid causes the pH of the water to decrease.
Contributed by: Ammar Ibrahim (December 2017)
Additional contributions by: Eitan Geva (University of Michigan)
Open content licensed under CC BY-NC-SA
Details
References
[1] Imperial College London. “Safe Use of Carbon Dioxide in Laboratories.” (Dec 4, 2017) www3.imperial.ac.uk/pls/portallive/docs/1/1391900.PDF.
[2] S. Bialkowski. “Carbon Dioxide and Carbonic Acid.” (Dec 4, 2017) ion.chem.usu.edu/~sbialkow/Classes/3650/Carbonate/Carbonic%20 Acid.html.
[3] Aqion. “Composite and True Carbonic Acid.” (Dec 4, 2017) www.aqion.de/site/145.
[4] C. Chieh. “The Ideal Gas Law.” University of Waterloo. (Dec 4, 2017) www.science.uwaterloo.ca/~cchieh/cact/c120/idealgas.html.
[5] D. C. Caldwell, R. J. Lewis, R. M. Shafstall and R. D. Johnson. “The Sublimation Rate of Dry Ice Packaged in Commonly Used Quantities by the Air Cargo Industry.” Defense Technical Information Center. (Dec 4, 2017) www.dtic.mil/cgi-bin/GetTRDoc?Location=U2&doc=GetTRDoc.pdf&AD=ADA461451.
[6] V. Ruz, M. M. Gonzalez, D. Winant, Z. Rodriguez and G. Van den Mooter, “Characterization of the Sublimation and Vapor Pressure of 2-(2-Nitrovinyl) Furan (G-0) Using Thermogravimetric Analysis: Effects of Complexation with Cyclodextrins.” Molecules, 20(8), 2015 pp. 15175–15191. doi:10.3390/molecules200815175.
Submission from the Compute-to-Learn course at the University of Michigan.
Snapshots
Permanent Citation