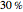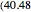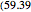Extractive Distillation Column to Separate Isopropyl Alcohol from Water

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
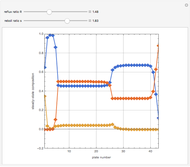
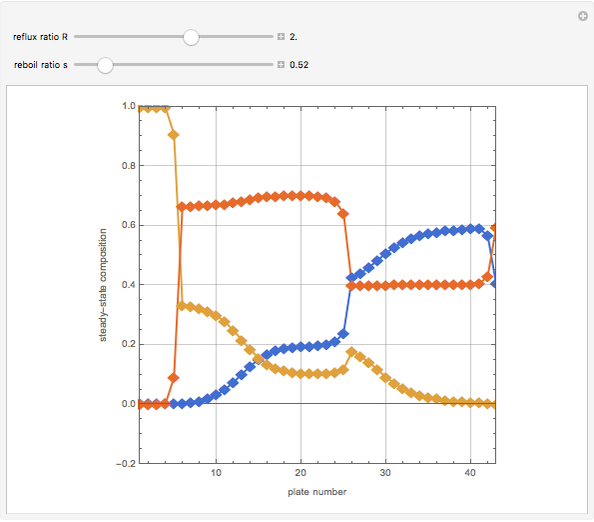
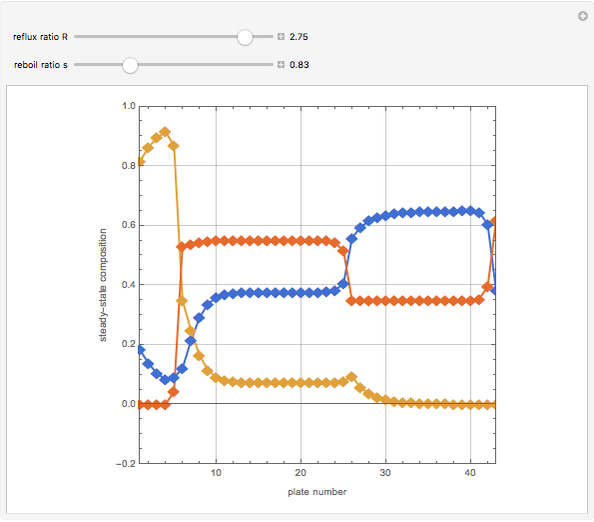
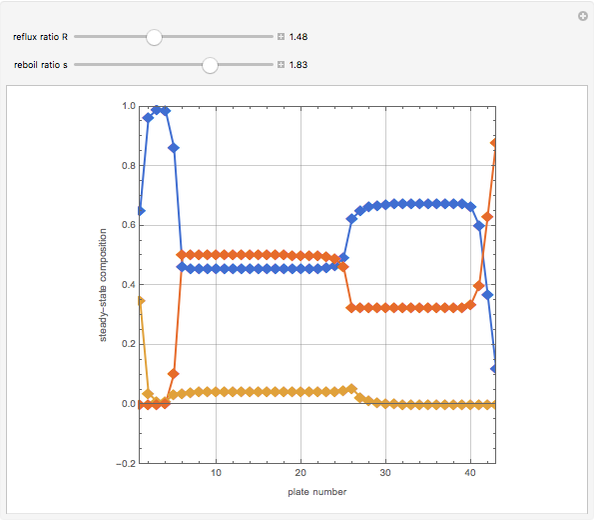
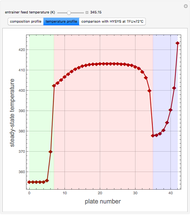
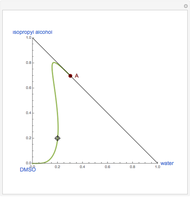
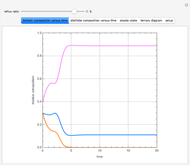
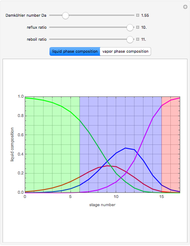
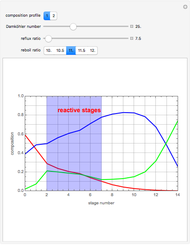
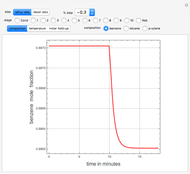
The binary mixture of water and isopropyl alcohol (isopropanol or propan-2-ol) presents a positive azeotrope at 1 atm. This azeotrope contains 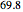 mole % isopropanol. It is possible to break this azeotrope using extractive distillation. Possible entrainers include: (1) 1,2-ethanediol or ethylene glycol, (2) DSMO (or dimethyl sulfoxide), (3)
mole % isopropanol. It is possible to break this azeotrope using extractive distillation. Possible entrainers include: (1) 1,2-ethanediol or ethylene glycol, (2) DSMO (or dimethyl sulfoxide), (3)  -methyl-2-pyrrolidone or NMP, (4)
-methyl-2-pyrrolidone or NMP, (4)  -methyl-2-piperidone, and (5)
-methyl-2-piperidone, and (5)  -methyl-6-caprolactam.
-methyl-6-caprolactam.
Contributed by: Housam Binous (December 2010)
(Kind Fahd University Petroleum & Minerals)
Open content licensed under CC BY-NC-SA
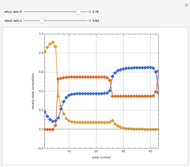
Snapshots
Details
For more information, see S. Arifin and I–L. Chien, Design and Control of Isopropyl Alcohol Dehydration via Homogeneous Azeotropic Distillation Using Dimethyl Sulfoxide as Extractive Agent.
Permanent Citation