Solubilization Model for Ionic Compounds

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
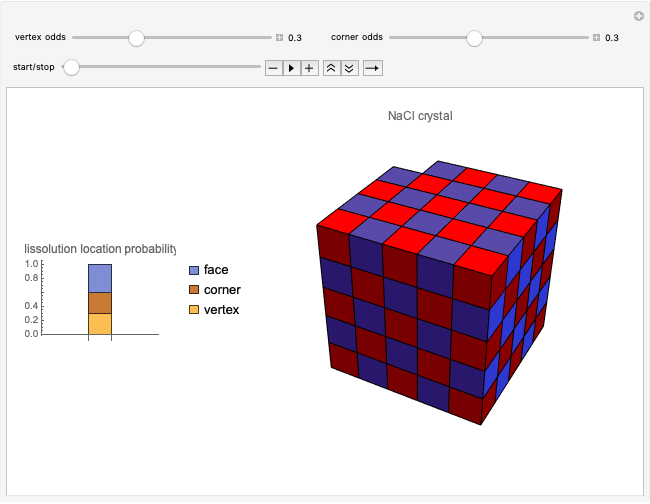
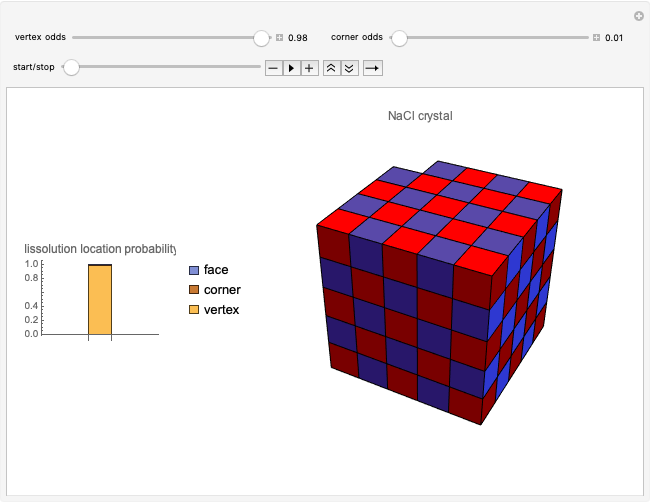
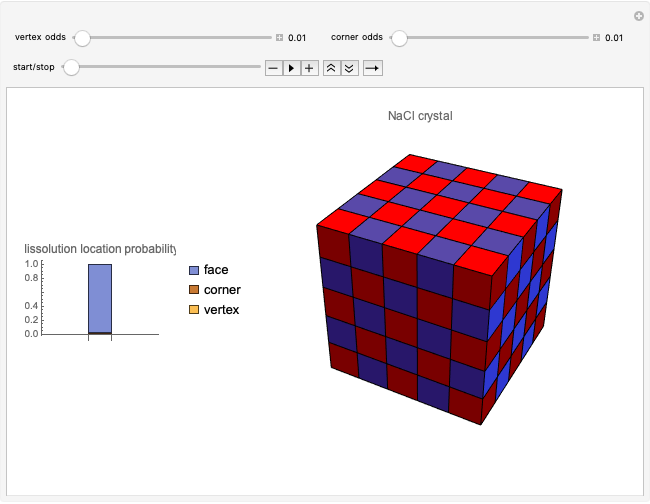
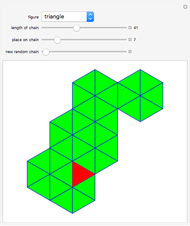
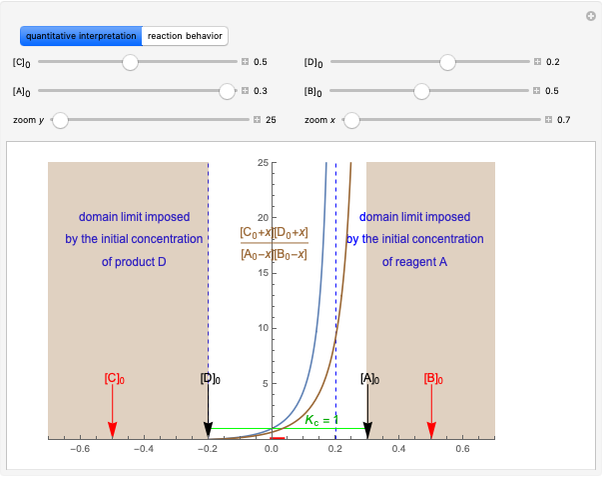
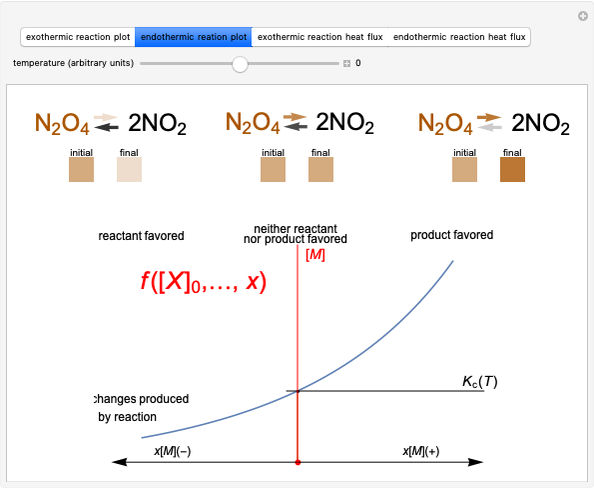
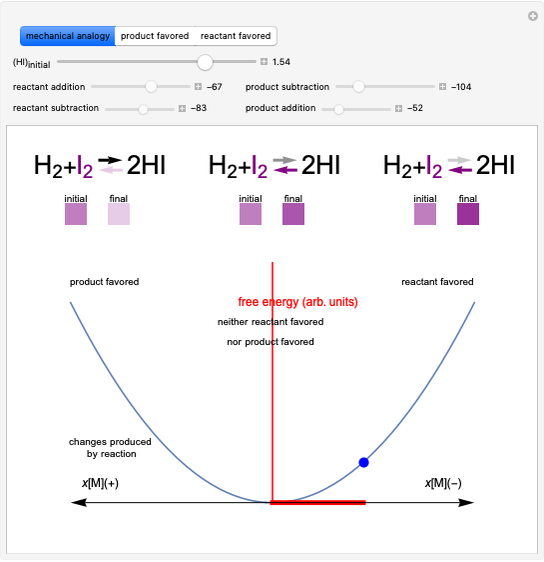
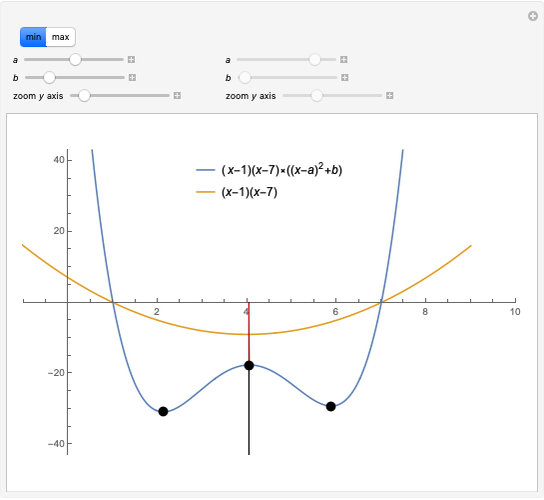
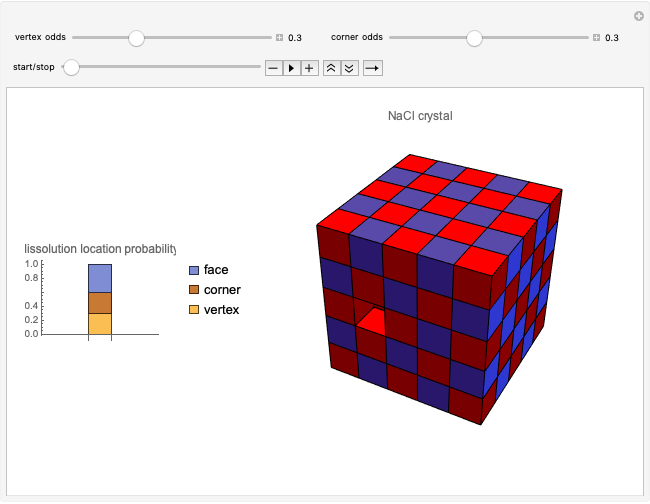
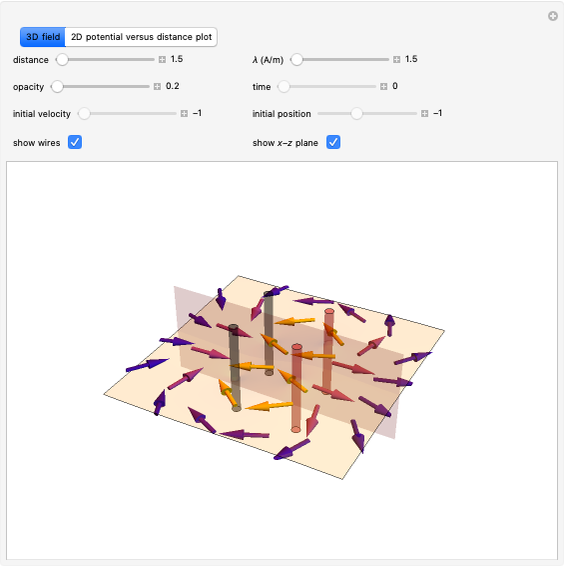
This Demonstration considers a model for the solubilization of an ionic compound in water.
[more]
Contributed by: D. Meliga and S. Z. Lavagnino (May 2019)
Additional contribution by: E. Conterosito
Open content licensed under CC BY-NC-SA
Snapshots
Details
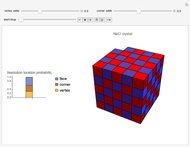
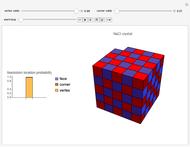
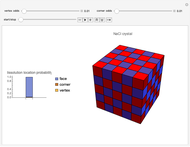
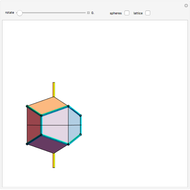
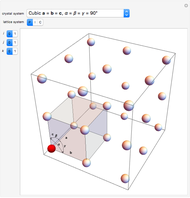
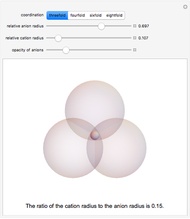
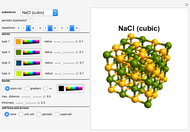
Snapshots 1, 2, 3: solubilization takes place mostly on the ions in the vertex/face/corner positions
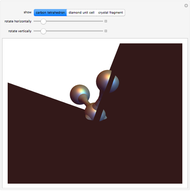
The following images are some photos taken during an experiment in our laboratory. We used a micropipette to inject water onto a single crystal of sodium chloride (∼0.1 mm side). The first photo shows a dazzling white crystal as a consequence of the scratches on the surface, and the first effect of the water is to smooth the surface, making it transparent. Subsequently, the solubilization starts.

Reference
[1] S. Coluccia, S. Lavagnino and L. Marchese, "The Hydroxylated Surface of MgO Powders and the Formation of Surface Sites," Materials Chemistry and Physics, 18(5–6), 1988 pp. 445–464. doi:10.1016/0254-0584(88)90016-8.
Permanent Citation