The Structure of Diamond

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
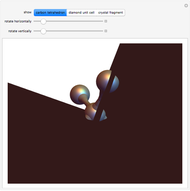
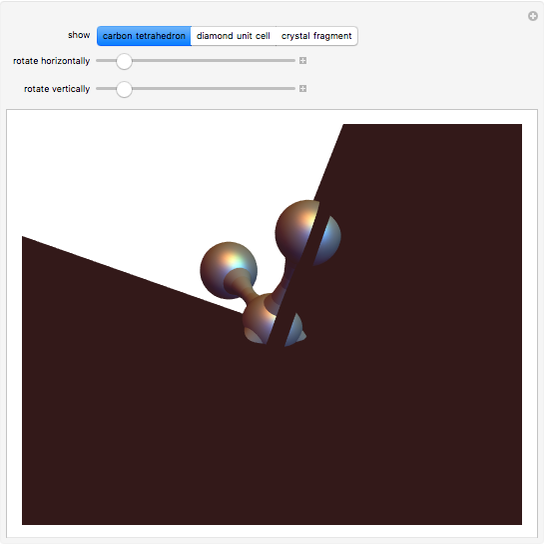
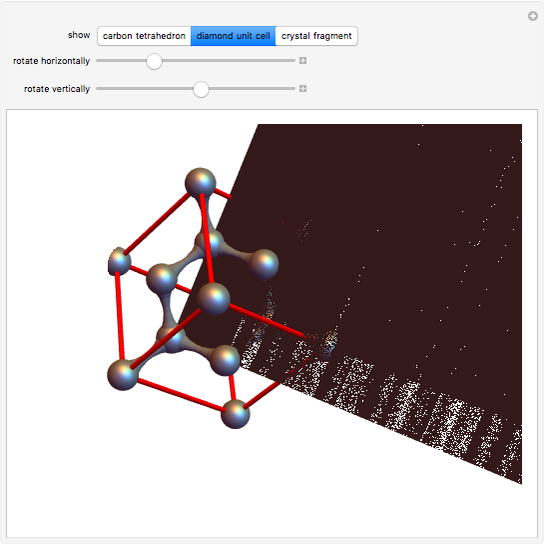
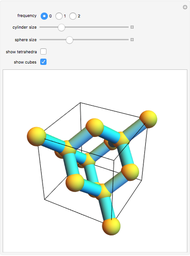
Diamond has the highest hardness and thermal conductivity of any bulk material while remaining an electrical insulator. The structure of diamond is based on a continuous network of tetrahedrally bonded carbon atoms in which extremely strong covalent bonds are formed between  -hybrid orbitals. The C–C bond length is 154.448 pm. The crystalline structure is of cubic symmetry with unit cell dimension
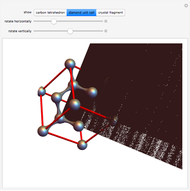
-hybrid orbitals. The C–C bond length is 154.448 pm. The crystalline structure is of cubic symmetry with unit cell dimension 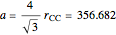 pm, containing eight carbon atoms per unit cell. The structure can be characterized as two interpenetrating face-centered cubic lattices, displaced by
pm, containing eight carbon atoms per unit cell. The structure can be characterized as two interpenetrating face-centered cubic lattices, displaced by  in each dimension.
in each dimension.
Contributed by: S. M. Blinder (April 2011)
Open content licensed under CC BY-NC-SA
Snapshots
Details
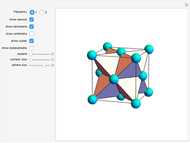
Snapshot 1: tetrahedral carbon array
Snapshot 2: unit cell viewed along a threefold axis
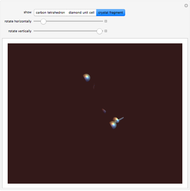
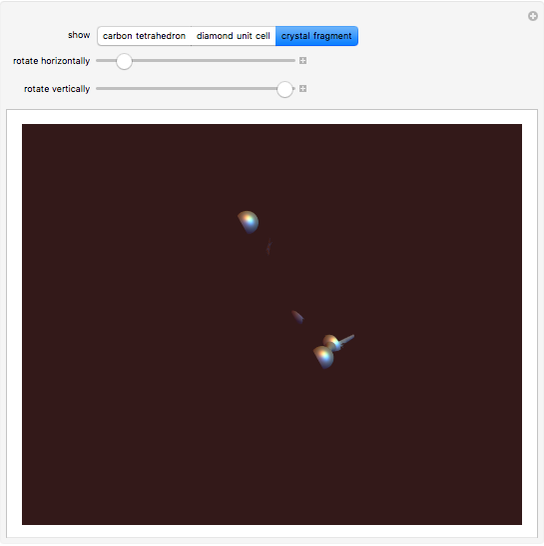
Snapshot 3: fragment of crystal containing 9 unit cells
For more information, see Diamond and Diamond Cubic.
Permanent Citation
"The Structure of Diamond"
http://demonstrations.wolfram.com/TheStructureOfDiamond/
Wolfram Demonstrations Project
Published: April 13 2011