Bohr's Orbits

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
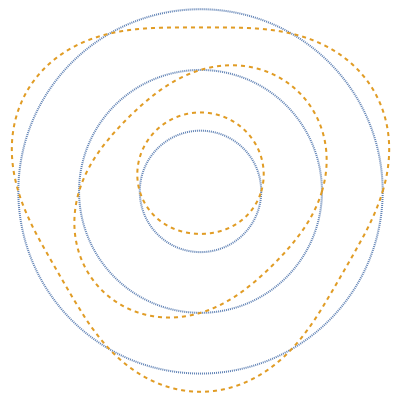
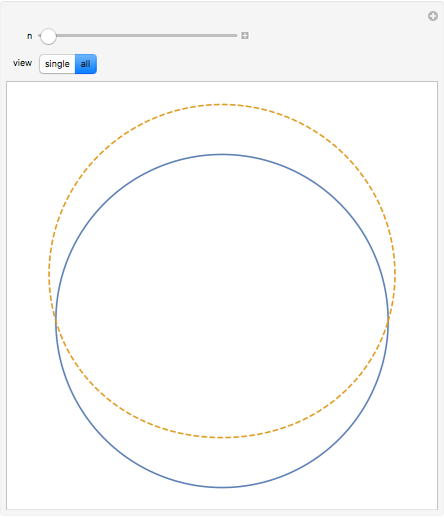
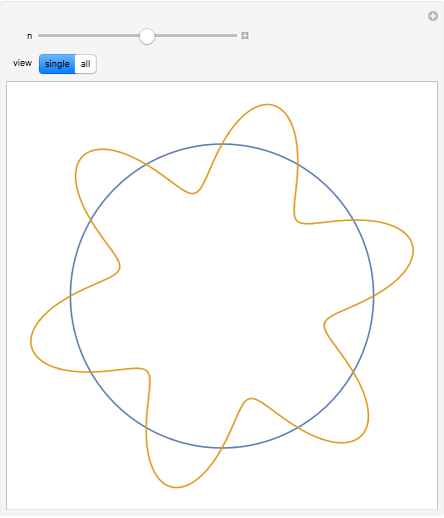
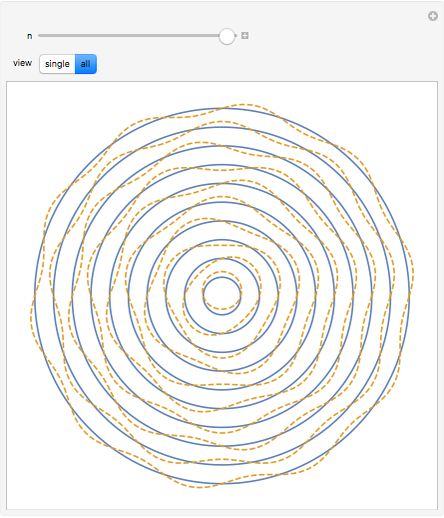
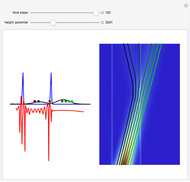
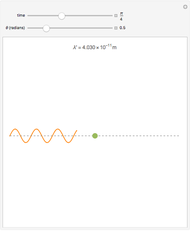
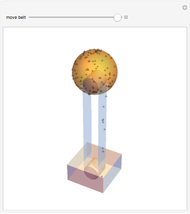
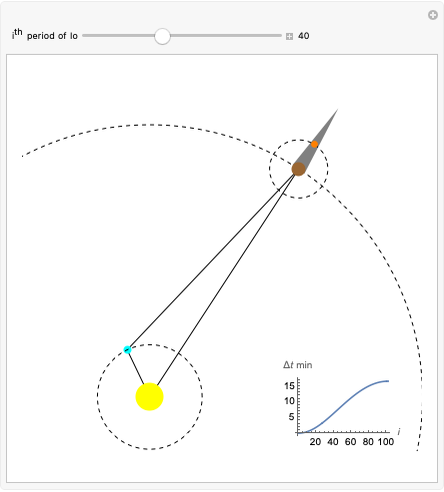
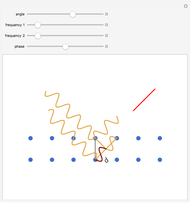
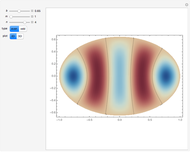
The plot shows stationary de Broglie waves in the Bohr model of the hydrogen atom. The electron, behaving as a wave, is stable in orbits containing an integer number of wavelengths. This model was an early precursor of the quantum theory.
Contributed by: Enrique Zeleny (March 2011)
Open content licensed under CC BY-NC-SA
Snapshots
Details
The de Broglie's relation is  , and the allowed wavelengths for a stable orbit obey the condition
, and the allowed wavelengths for a stable orbit obey the condition  , where
, where  is the wavelength,
is the wavelength,  is the radius, and
is the radius, and  .
.
Permanent Citation
"Bohr's Orbits"
http://demonstrations.wolfram.com/BohrsOrbits/
Wolfram Demonstrations Project
Published: March 7 2011