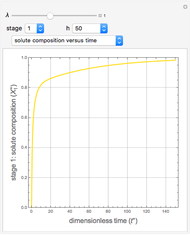
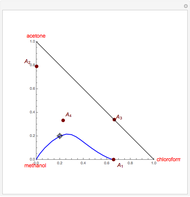
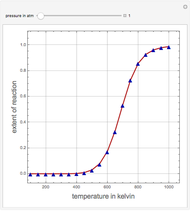
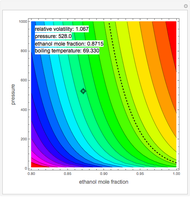
Breaking the Acetone-Methanol Azeotrope with Different Extraction Solvents

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
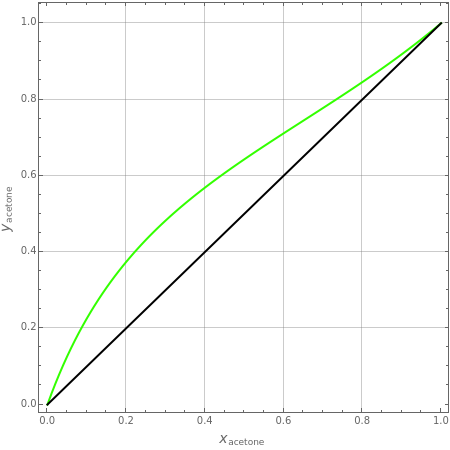
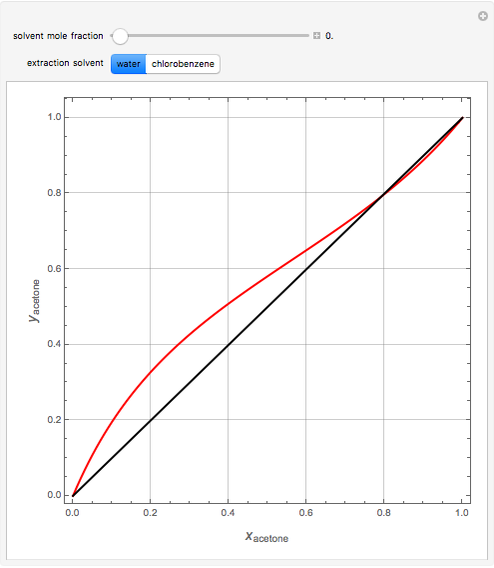
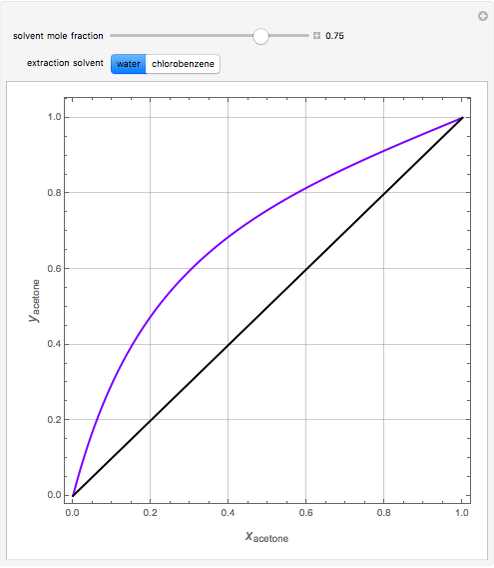
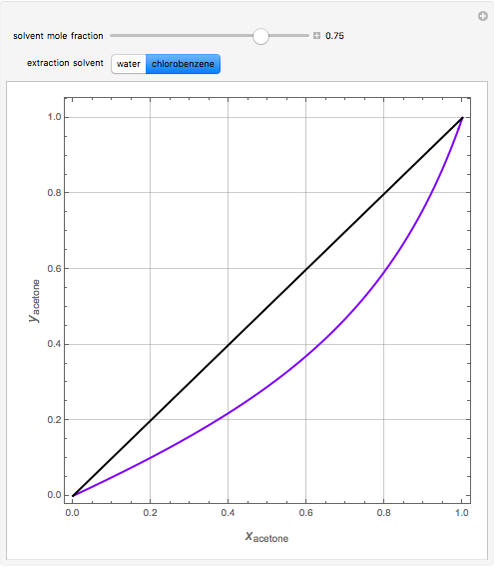
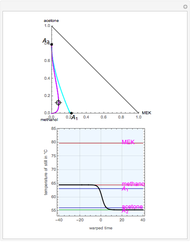
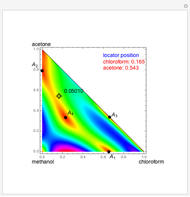
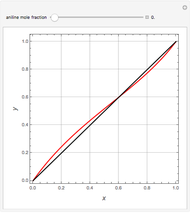
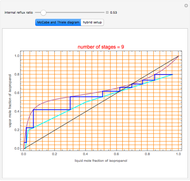
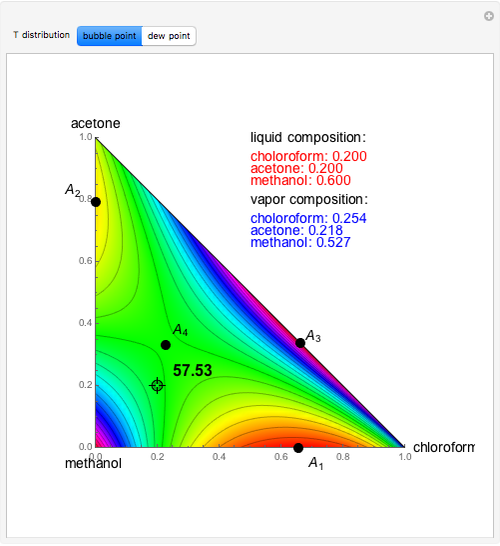
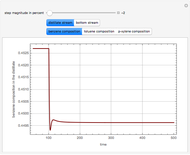
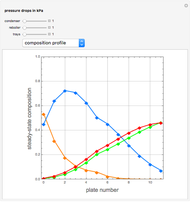
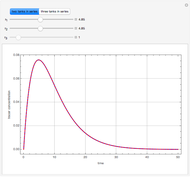
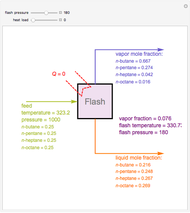
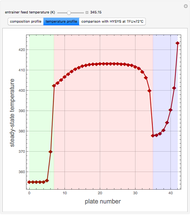
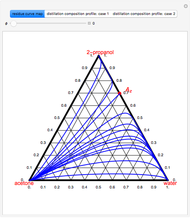
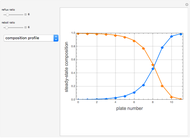
Separation of a binary mixture composed of acetone (b.p.  ) and methanol (b.p.
) and methanol (b.p.  ) using a distillation column is difficult because of the existence of a positive or minimum-boiling azeotrope (b.p.
) using a distillation column is difficult because of the existence of a positive or minimum-boiling azeotrope (b.p.  , composition
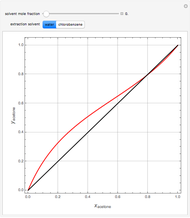
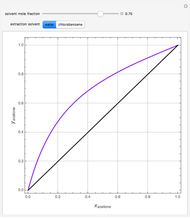
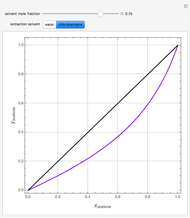
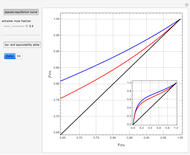
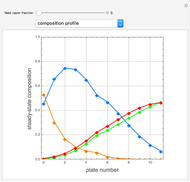
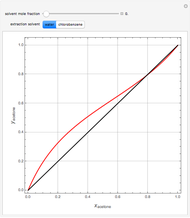
, composition  ). For this reason, one has to use more complex distillation methods such as extractive distillation, which employs various kinds of entrainers or extraction solvents such as water or chlorobenzene.
). For this reason, one has to use more complex distillation methods such as extractive distillation, which employs various kinds of entrainers or extraction solvents such as water or chlorobenzene.
Contributed by: Housam Binous and Naim Faqir (August 2012)
Open content licensed under CC BY-NC-SA
Snapshots
Details
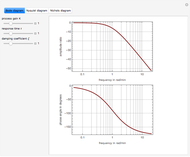
The vapor-liquid equilibrium (VLE) behavior is described by the modified Raoult’s law with activity coefficients predicted by the Wilson model [2].
References
[1] W. L. Luyben and I.-L. Chien, Design and Control of Distillation Systems for Separating Azeotropes, Hoboken, NJ: Wiley, 2010.
[2] G. M. Wilson, "Vapor-Liquid Equilibrium XI: A New Expression for the Excess Free Energy of Mixing," Journal of the American Chemical Society, 86(2), 1964 pp. 127–130. pubs.acs.org/doi/abs/10.1021/ja01056a002.