Breaking the Azeotrope between Methanol and Acetone with an Entrainer

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
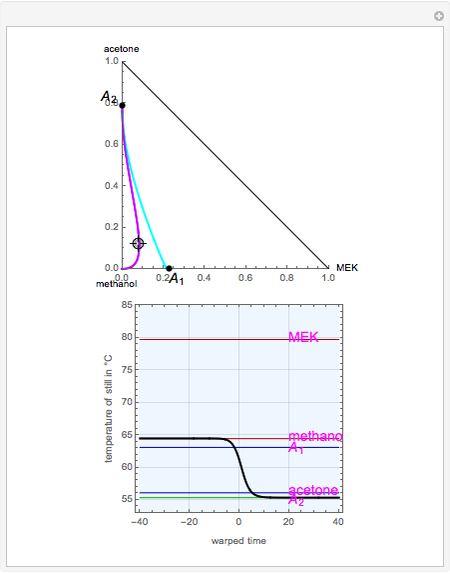
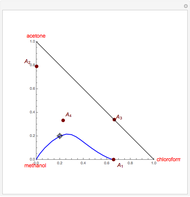
The binary mixture of acetone (boiling point  ) and methanol (boiling point
) and methanol (boiling point  ) is difficult to separate by distillation because of the presence of a minimum boiling azeotrope (
) is difficult to separate by distillation because of the presence of a minimum boiling azeotrope ( mole % acetone at
mole % acetone at  ). This Demonstration shows that MEK (boiling point
). This Demonstration shows that MEK (boiling point  ) cannot be used as an entrainer to break this azeotrope at 1 atm. Indeed, MEK forms with methanol a minimum boiling azeotrope (
) cannot be used as an entrainer to break this azeotrope at 1 atm. Indeed, MEK forms with methanol a minimum boiling azeotrope (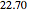 mole % MEK at
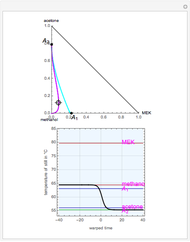
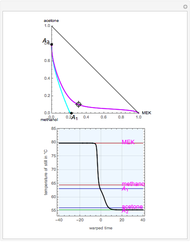
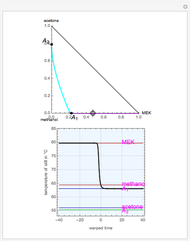
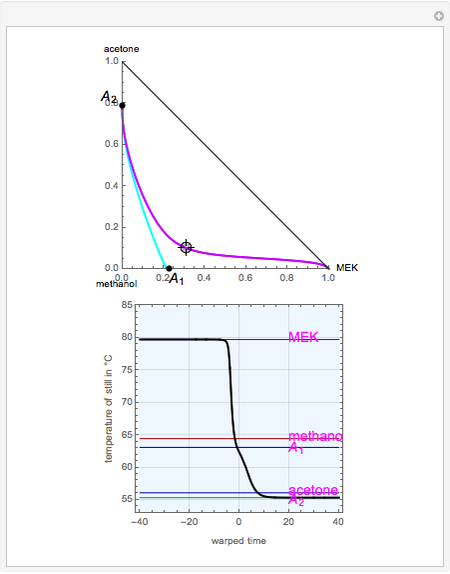
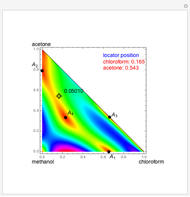
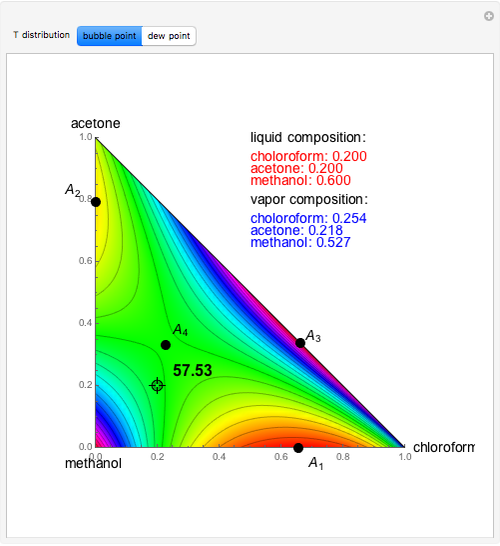
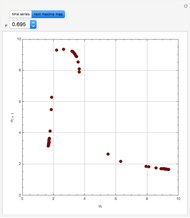
mole % MEK at  ), and a distillation boundary (shown in cyan) divides the composition space into two distillation regions. Methanol and acetone are separated by this boundary. The temperature versus warped time clearly shows the nature of the pure components and azeotropes.
), and a distillation boundary (shown in cyan) divides the composition space into two distillation regions. Methanol and acetone are separated by this boundary. The temperature versus warped time clearly shows the nature of the pure components and azeotropes. 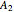 , indicated by a green line, is an unstable node. Acetone and
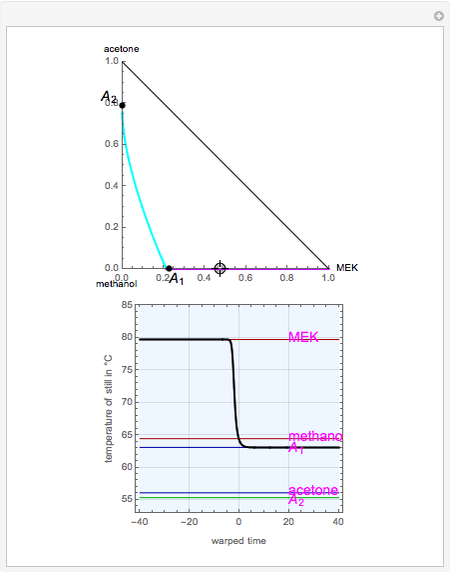
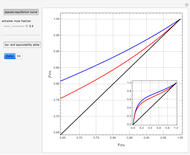
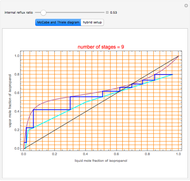
, indicated by a green line, is an unstable node. Acetone and  , indicated by a blue line, are saddle points. MEK and methanol, indicated by a red line, are stable points. Two snapshots show what happens if you choose a simple binary mixture. Of course, for a ternary mixture, you have to select a locator position inside the triangular diagram.
, indicated by a blue line, are saddle points. MEK and methanol, indicated by a red line, are stable points. Two snapshots show what happens if you choose a simple binary mixture. Of course, for a ternary mixture, you have to select a locator position inside the triangular diagram.
Contributed by: Housam Binous (March 2011)
(Kind Fahd University Petroleum & Minerals)
Open content licensed under CC BY-NC-SA
Snapshots
Details
Reference
[1] V. Julka, M. Chiplunkar, and L. O'Young, "Selecting Entrainers for Azeotropic Distillation," Chemical Engineering Progress, 105(3), 2009 pp. 47–53.