Limiting Reagent for the Reaction of Hydrogen and Oxygen to Form Water

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
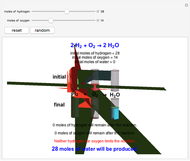
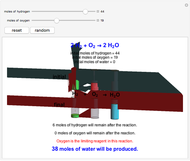
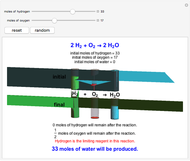
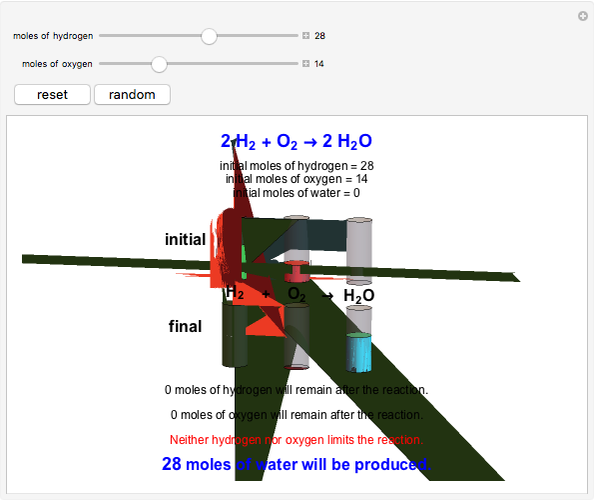
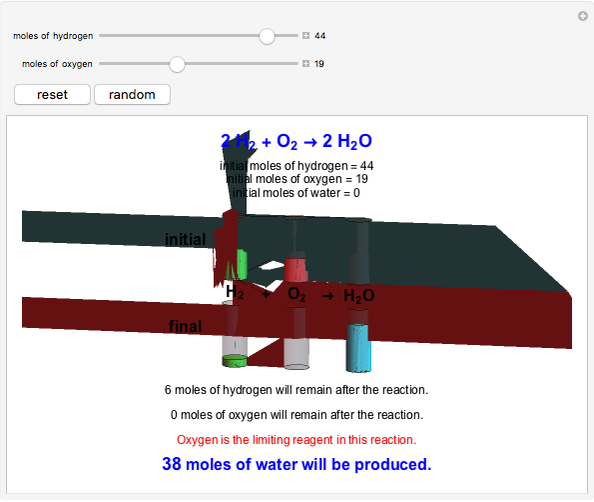
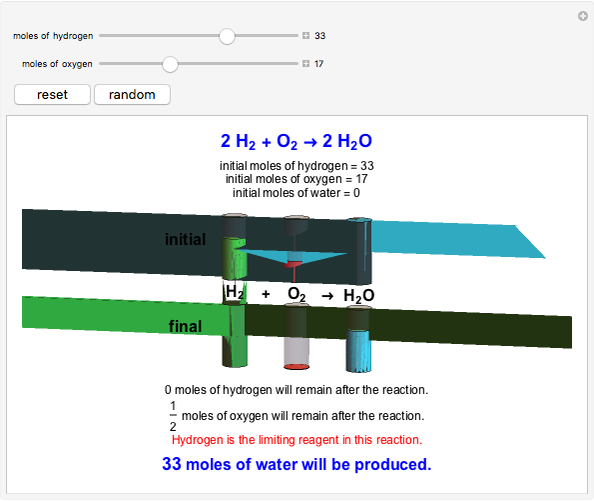
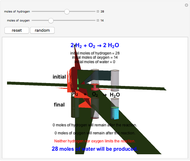
This Demonstration shows the effect of a limiting reagent during the formation of a product, given varying amounts of reactants. The two reactants, hydrogen and oxygen, are shown to yield the product, water, assuming an appropriate catalyst is present. The first set of cylinders shows the number of moles of each reagent, and the second set shows the final number of moles after the reaction has run to completion. Either of the reactants can be the limiting reagent, based on the initial number of moles and the stoichiometry of the reaction.
Contributed by: Sameera Raziuddin, Nabeela Mohideen, David Dimitroff, Nicholas Miljevic, Michael Iqbal, Christopher Hilbrich, and Kelly Stanley (December 2012)
With additional contributions by: Nelson DeLeon and Linda Wozniewski
Open content licensed under CC BY-NC-SA
Snapshots
Details
Snapshot 1: neither of the reactants limits the reaction
Snapshot 2: oxygen is the limiting reagent in this situation; there are 0 moles of oxygen remaining after the reaction proceeds to produce water
Snapshot 3: hydrogen is the limiting reagent in this situation; this is shown by the 0 moles of hydrogen in the final state
Permanent Citation