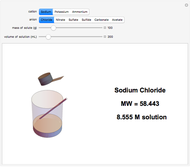
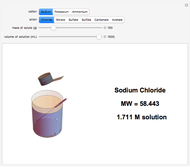
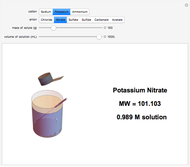
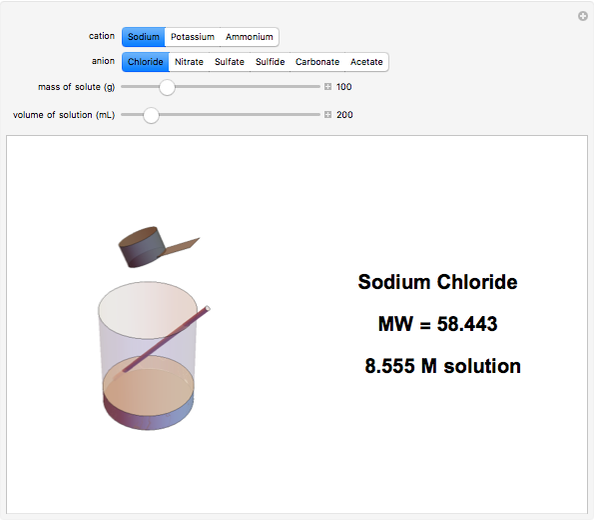
Molarity of Aqueous Salt Solutions

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
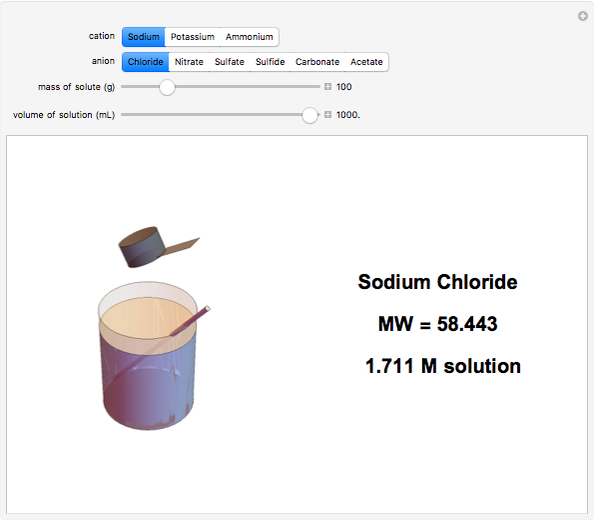
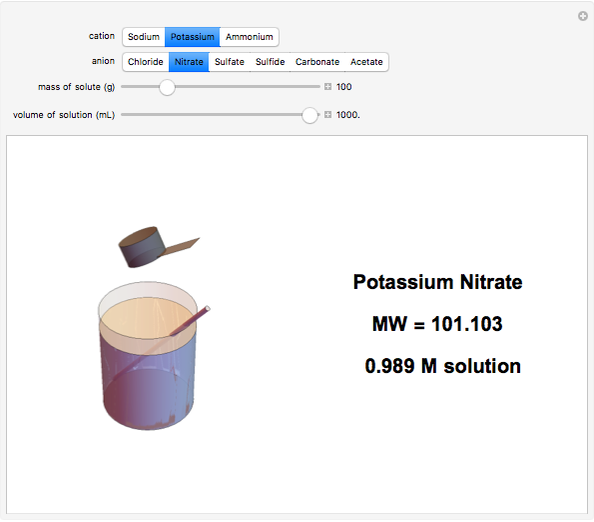
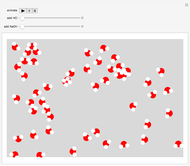
Input the mass of solute and the volume of the resulting solution to calculate the molarity (M, in moles/liter) of several common water-soluble salts. The number of moles equals the mass divided by the molecular weight MW. Generally, since the volume of the solution is only very slightly changed by addition of solute, it is usually a sufficiently accurate approximation to compute molarity as moles of solute divided by volume of solvent.
[more]
Contributed by: S. M. Blinder (July 2008)
Open content licensed under CC BY-NC-SA
Snapshots
Details
detailSectionParagraphPermanent Citation
"Molarity of Aqueous Salt Solutions"
http://demonstrations.wolfram.com/MolarityOfAqueousSaltSolutions/
Wolfram Demonstrations Project
Published: July 7 2008