Michaelis-Menten Kinetics for Hydrogen Peroxide-Catalase Reaction

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
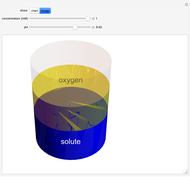
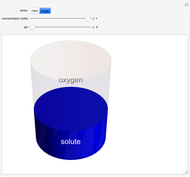
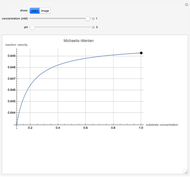
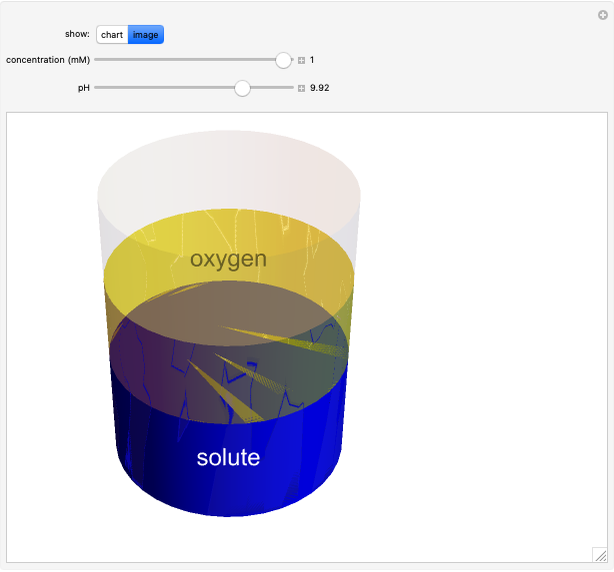
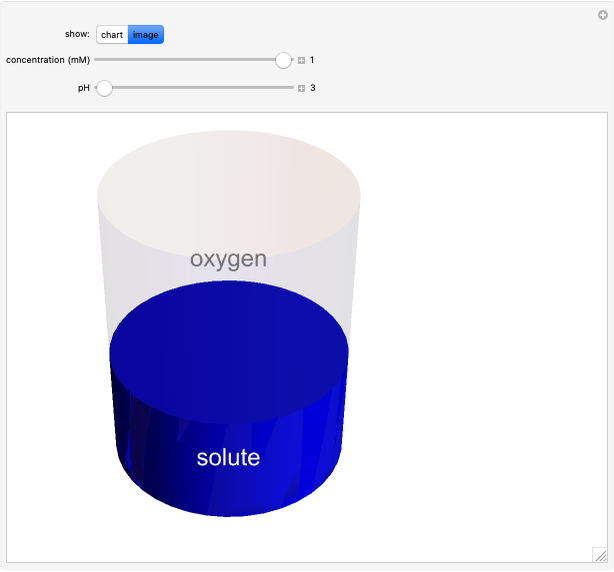
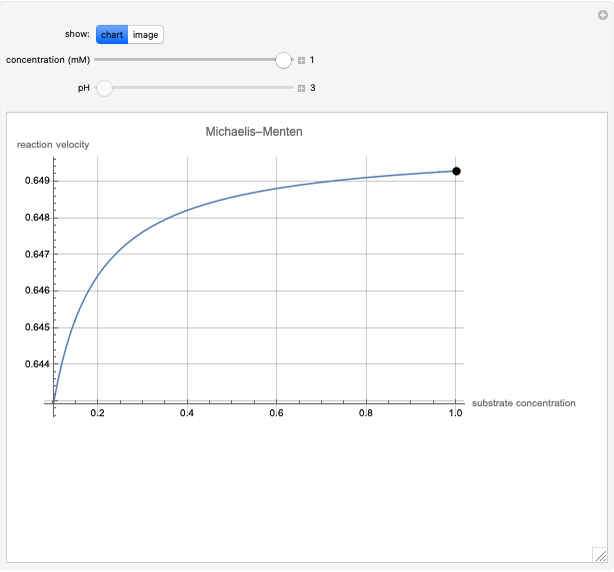
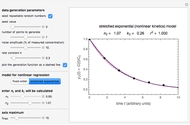
Catalase is an important enzyme found in the human liver. The Michaelis–Menten equation is one of the best-known models for representing enzyme kinetics. Using this chart, you can see how a change in concentration for a particular substrate (in this case, hydrogen peroxide) affects the reaction rate.
[more]
Contributed by: Charlotte Geoghegan, Shantellie Hickinson and Joseph Keating (August 2022)
With additional contributions by: Dalia Hassan, Yifan Lai and Kristina M. Lenn
Open content licensed under CC BY-NC-SA
Snapshots
Details
Reference
[1] P. Jones and A. Suggett, "The Catalase–Hydrogen Peroxide System. Kinetics of Catalatic Action at High Substrate Concentrations," Biochemical Journal, 110(4), 1968 pp. 617–620. doi:10.1042/bj1100617.
Permanent Citation