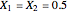Methyl tert-butyl ether, also known as MTBE, is a chemical compound with molecular formula  . MTBE, as well as TAME (tert-amyl butyl ether), are gasoline additives used as oxygenates to raise the octane number.
. MTBE, as well as TAME (tert-amyl butyl ether), are gasoline additives used as oxygenates to raise the octane number.
The following etherification reaction produces MTBE: isobutene + MeOH ⇌ MTBE. The reacting mixture contains  -butane, an inert component, in addition to methanol (MeOH), isobutene, and MTBE. The reaction extent is limited, with an equilibrium constant taken equal to 49.0.
-butane, an inert component, in addition to methanol (MeOH), isobutene, and MTBE. The reaction extent is limited, with an equilibrium constant taken equal to 49.0.
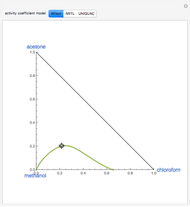
Vapor-liquid equilibrium is predicted using the Wilson model and the modified Raoult's law. Deviation from ideal behavior in the gas-phase is not taken into account to keep the calculation simple and to compare with available data in the literature (see [1] and [2]).
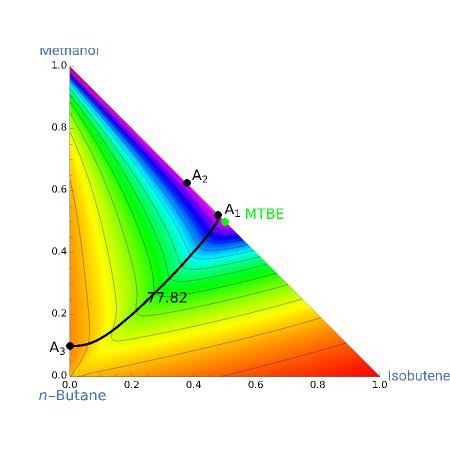
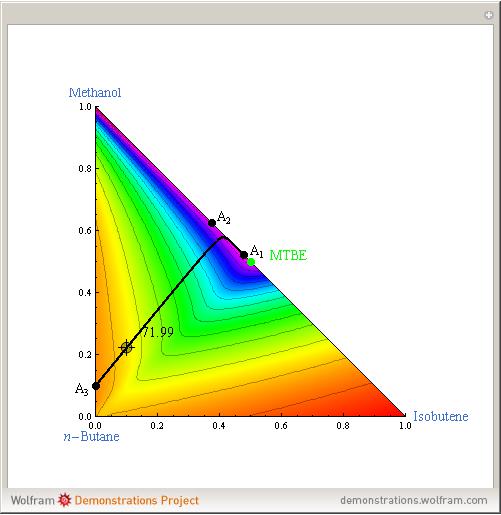
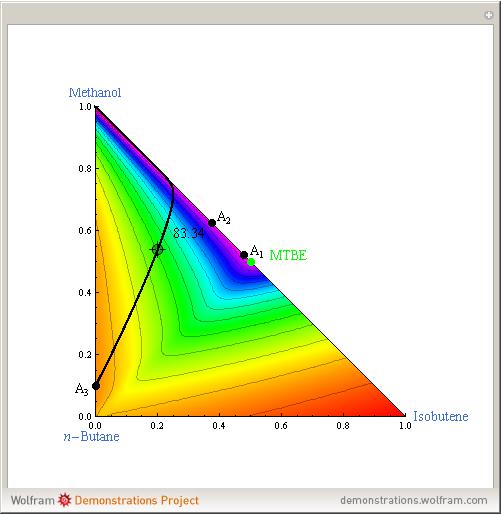
Azeotropes between MeOH and isobutene and MTBE and MeOH are reacted away. The azeotrope  between MeOH and
between MeOH and  -butane is an unstable node containing 9.88 mole % MeOH. In addition, there are two reactive azeotropes: (1)
-butane is an unstable node containing 9.88 mole % MeOH. In addition, there are two reactive azeotropes: (1)  close to pure MTBE, corresponding to a stable node; (2)
close to pure MTBE, corresponding to a stable node; (2)  , corresponding to a saddle point. The coordinates of the two reactive azeotropes, which contain no
, corresponding to a saddle point. The coordinates of the two reactive azeotropes, which contain no  -butane, are
-butane, are
 and
and  ,
,
 and
and  .
.
These coordinates of the reactive azeotropes are in agreement with values given in the literature (see [1] and [2]).
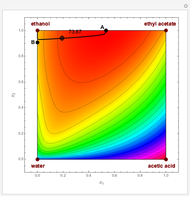
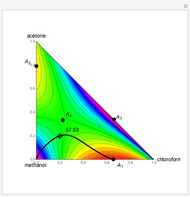
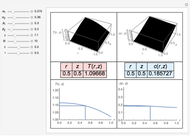
This Demonstration shows plots of the residue curve passing through a user-specified location in the triangular diagram. The  and
and  axes of the figure are related to liquid phase compositions by
axes of the figure are related to liquid phase compositions by
 and
and  ,
,
where  ,
,  , and
, and  are the mole fractions of isobutene, MeOH and MTBE, respectively.
are the mole fractions of isobutene, MeOH and MTBE, respectively.
The position of pure MTBE, shown by a green dot in the diagram, is given by 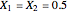 .
.
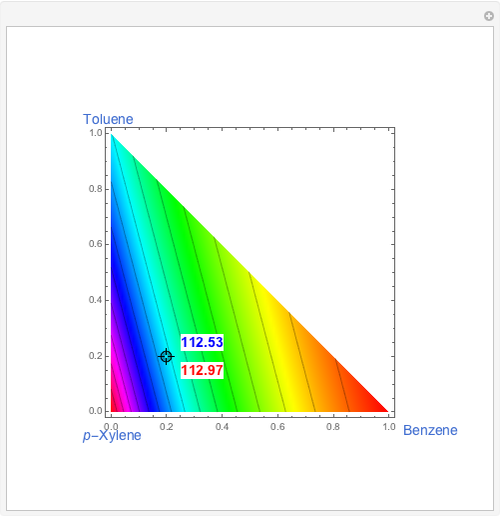
The bubble temperature distribution is superimposed on the residue curve plot and clearly indicates the nature of the azeotropes (unstable node, saddle point, or stable node). For example, a stable node corresponds to high bubble temperatures (shown in magenta in the diagram) while an unstable node will be found in the yellow regions associated with low bubble temperatures. Finally, the temperature at the locator's position and iso-temperature curves are displayed in the same diagram.
[less]

 . MTBE, as well as TAME (tert-amyl butyl ether), are gasoline additives used as oxygenates to raise the octane number.
. MTBE, as well as TAME (tert-amyl butyl ether), are gasoline additives used as oxygenates to raise the octane number.