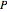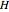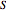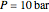Residual Functions for the SRK and PR Equations of State

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
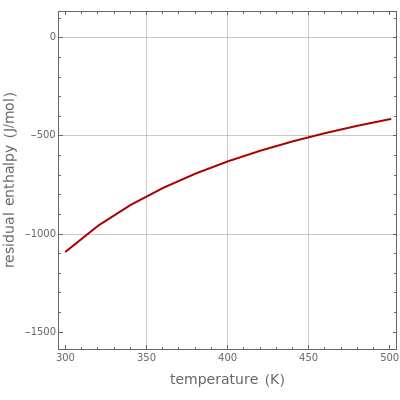
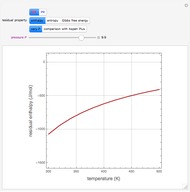
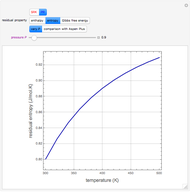
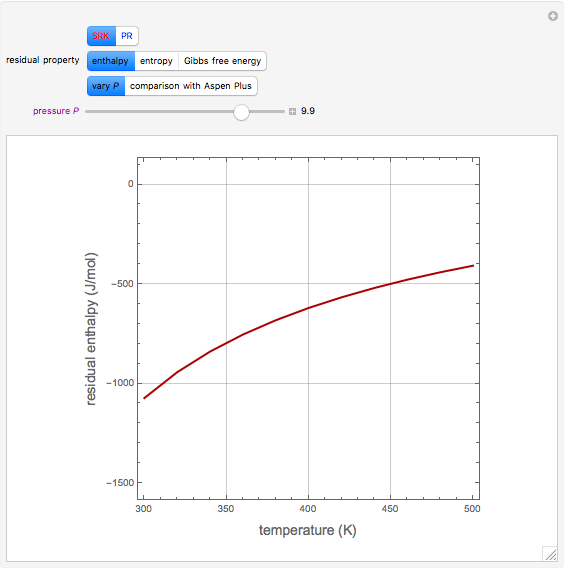
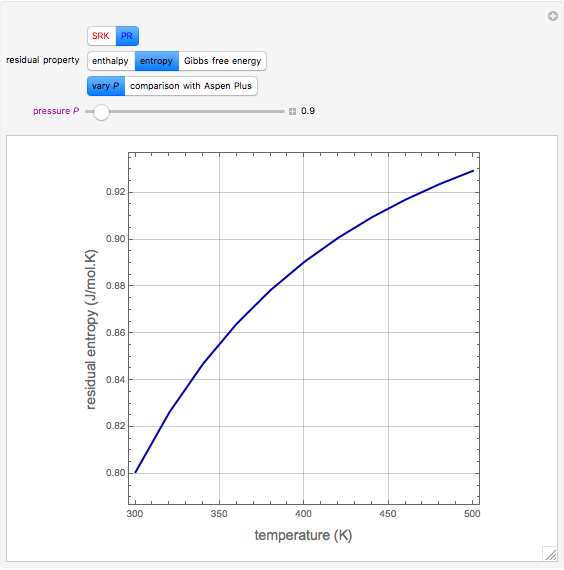
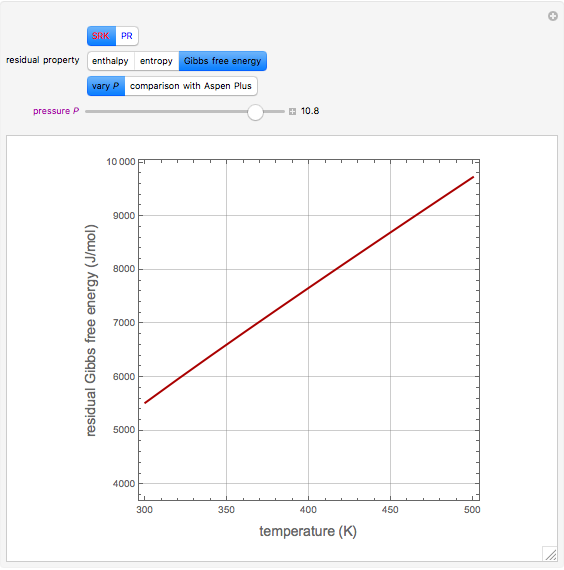
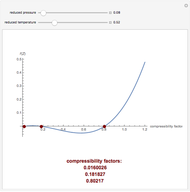
In the gas phase, deviation from ideal behavior is taken into account by introducing residual functions, of the form  . As pressure approaches zero, the gas behaves like an ideal gas, so that
. As pressure approaches zero, the gas behaves like an ideal gas, so that  .
.
Contributed by: Housam Binous and Ahmed Bellagi (December 2016)
Open content licensed under CC BY-NC-SA
Snapshots
Details
References
[1] J. M. Smith, H. C. Van Ness and M. M. Abbott, Introduction to Chemical Engineering Thermodynamics, 7th ed., Boston: McGraw-Hill, 2005.
[2] J. R. Elliott and C. T. Lira, Introductory Chemical Engineering Thermodynamics, 2nd ed., Englewood Cliffs, NJ: Prentice Hall International Editions, 2012.
[3] Aspentech. "Aspen Plus." (Dec 19, 2016) www.aspentech.com/products/engineering/aspen-plus.
Permanent Citation