Liquid-Liquid Equilibrium for the 1-Butanol-Water System

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
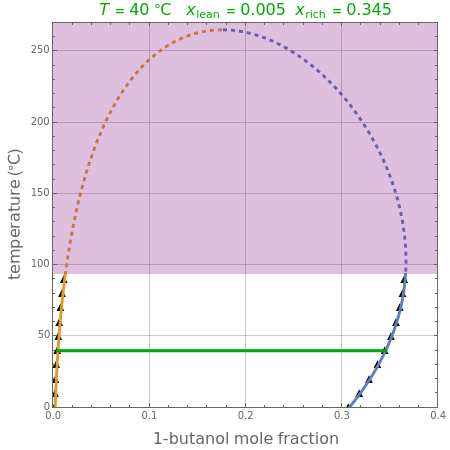
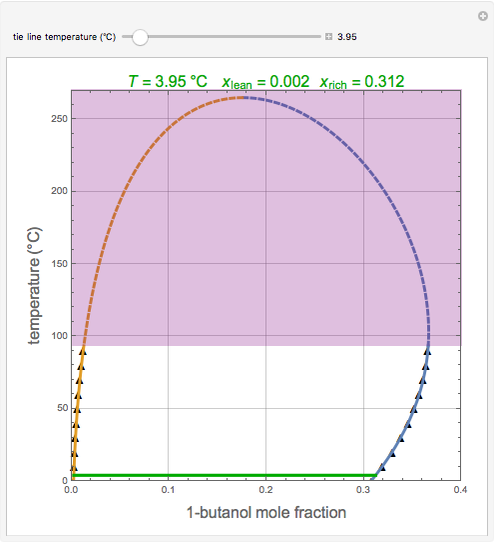
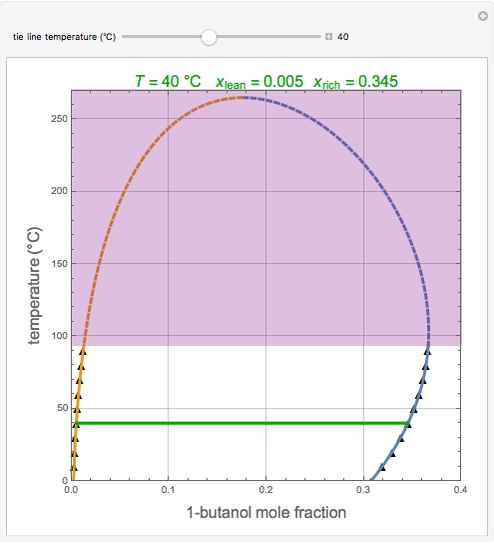
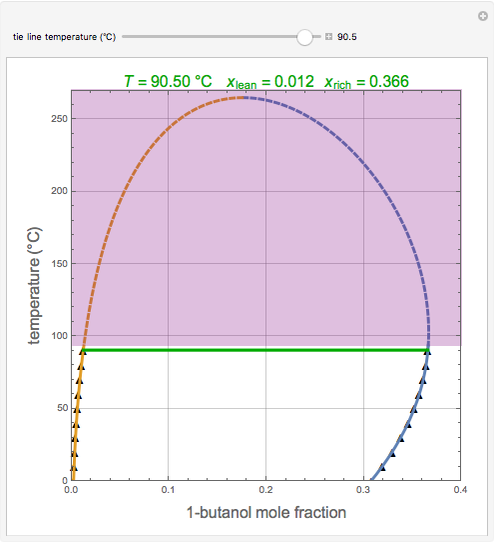
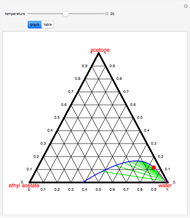
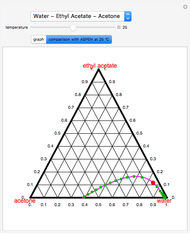
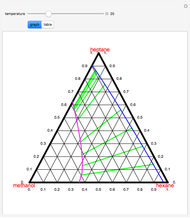
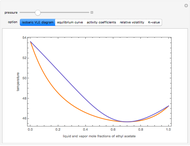
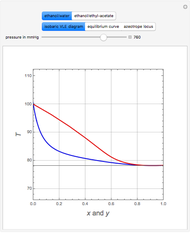
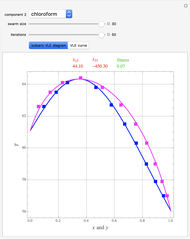
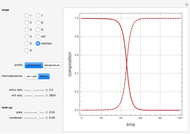
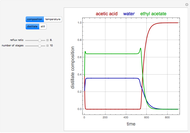
Consider the partially miscible binary mixture composed of  -butanol and water at 101.325 °C. The nonrandom two-liquid (NRTL) model developed by Renon and Prausnitz [1, 2] is adequate for determining liquid-liquid equilibrium (LLE) data. Since this mixture presents a heteroazeotrope with a boiling point of 93.49 °C, we consider only temperatures below this threshold value. The magenta-shaded region indicates where the validity of our simulation fails. Indeed, for
-butanol and water at 101.325 °C. The nonrandom two-liquid (NRTL) model developed by Renon and Prausnitz [1, 2] is adequate for determining liquid-liquid equilibrium (LLE) data. Since this mixture presents a heteroazeotrope with a boiling point of 93.49 °C, we consider only temperatures below this threshold value. The magenta-shaded region indicates where the validity of our simulation fails. Indeed, for  °C we have, in addition to the two liquid phases, a third vapor phase.
°C we have, in addition to the two liquid phases, a third vapor phase.
Contributed by: Housam Binous and Ahmed Bellagi (December 2016)
Open content licensed under CC BY-NC-SA
Snapshots
Details
References
[1] J. M. Smith, H. C. Van Ness and M. M. Abbott, Introduction to Chemical Engineering Thermodynamics, 7th ed., Boston: McGraw-Hill, 2005.
[2] H. Renon and J. M. Prausnitz, "Local Compositions in Thermodynamic Excess Functions for Liquid Mixtures," AIChE Journal, 14(1), 1968 pp. 135–144. doi:10.1002/aic.690140124.
[3] Aspentech. "Aspen-HYSYS." (Dec 15, 2016) www.aspentech.com/products/aspen-hysys.
Permanent Citation
"Liquid-Liquid Equilibrium for the 1-Butanol-Water System"
http://demonstrations.wolfram.com/LiquidLiquidEquilibriumForThe1ButanolWaterSystem/
Wolfram Demonstrations Project
Published: December 16 2016