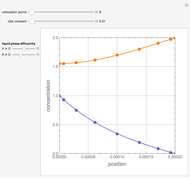
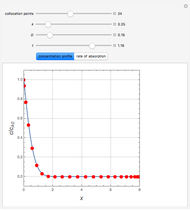
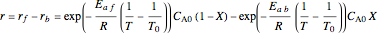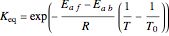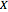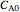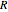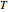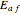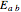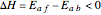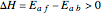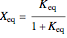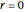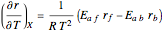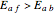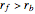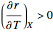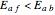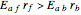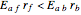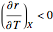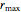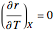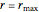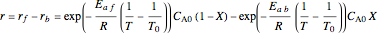 ,
,
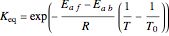 ,
,
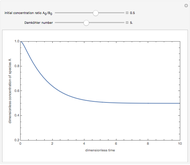
where 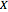 is the conversion fraction,
is the conversion fraction, 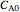 is the inlet concentration (taken to be 10 moles/liter),
is the inlet concentration (taken to be 10 moles/liter), 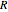 is the universal gas constant (1.987 cal/mol K), and
is the universal gas constant (1.987 cal/mol K), and 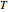 is the temperature (in kelvin).
is the temperature (in kelvin).
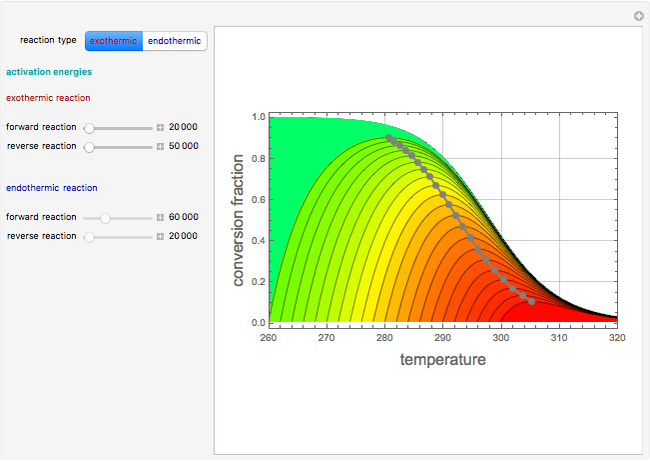
Use the sliders to vary the activation energies for the forward (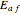 ) and reverse (
) and reverse (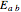 ) reactions. Both of these activation energies are expressed in cal/mol.
) reactions. Both of these activation energies are expressed in cal/mol.
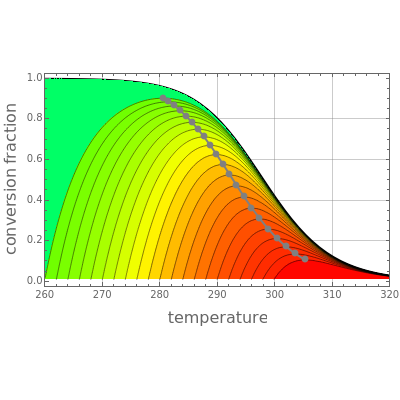
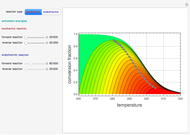
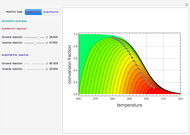
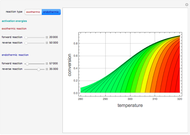
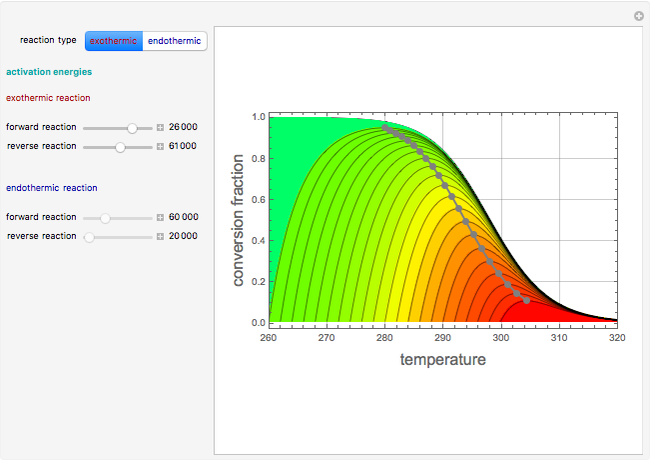
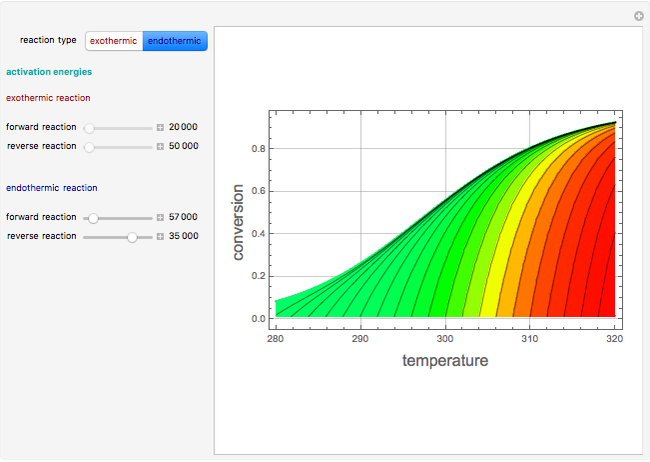
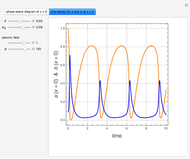
This Demonstration plots the contour lines for the reaction rate  either for exothermic reactions
either for exothermic reactions 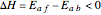 or for endothermic reactions
or for endothermic reactions 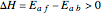 . The equilibrium conversion
. The equilibrium conversion 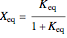 is plotted versus the temperature (see the black curve, for which we have
is plotted versus the temperature (see the black curve, for which we have 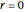 ). It can be easily shown that
). It can be easily shown that 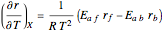 . Thus, for endothermic reactions,
. Thus, for endothermic reactions, 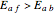 and
and 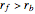 , so
, so 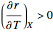 and
and  will increase monotonically with
will increase monotonically with 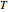 if you move along a horizontal line (i.e. at a constant conversion fraction). On the other hand, for exothermic reactions we have
if you move along a horizontal line (i.e. at a constant conversion fraction). On the other hand, for exothermic reactions we have 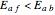 , thus we have along a horizontal line (i.e. at a constant value of
, thus we have along a horizontal line (i.e. at a constant value of 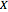 ): (1) at low temperature
): (1) at low temperature 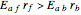 and
and 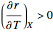 ; and (2) at higher temperature
; and (2) at higher temperature 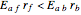 and
and 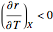 . In conclusion, for an exothermic reaction, the reaction rate
. In conclusion, for an exothermic reaction, the reaction rate  initially increases with increasing
initially increases with increasing 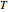 , reaches a maximum value
, reaches a maximum value 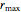 when
when 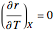 , then starts to decrease until it reaches the equilibrium conversion curve where
, then starts to decrease until it reaches the equilibrium conversion curve where 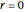 . The loci of the points where
. The loci of the points where 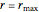 are indicated by the gray dots and curve.
are indicated by the gray dots and curve.
[less]

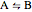 with the reaction rate
with the reaction rate  and the equilibrium constant
and the equilibrium constant 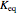 given by:
given by: