Rutherford Scattering

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
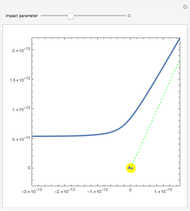
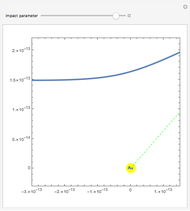
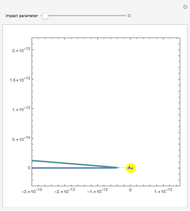
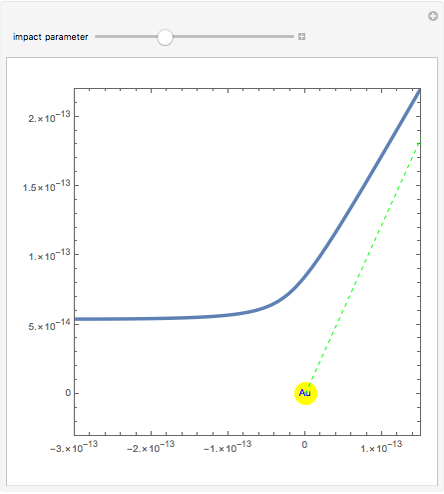
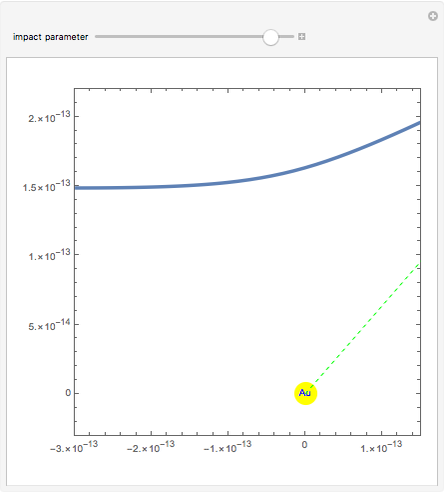
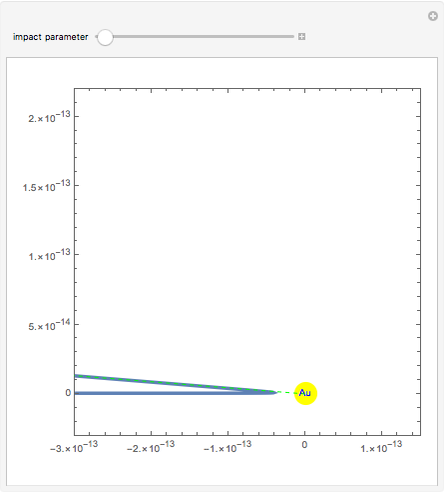
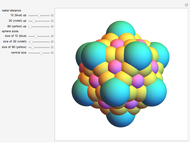
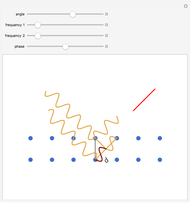
Rutherford's classic experiment consisted in bombarding a thin gold foil with  particles (helium nuclei). He expected that some particles would be slightly deflected by the positive charge inside the atoms. Most went through without much change, some were deflected, and surprisingly, some came straight back. This led Rutherford to conclude that the positive charge must be concentrated in a very small volume called the nucleus and not across the entire atom, with a typical radius of the order of
particles (helium nuclei). He expected that some particles would be slightly deflected by the positive charge inside the atoms. Most went through without much change, some were deflected, and surprisingly, some came straight back. This led Rutherford to conclude that the positive charge must be concentrated in a very small volume called the nucleus and not across the entire atom, with a typical radius of the order of  m.
m.
Contributed by: Enrique Zeleny (June 20)
Open content licensed under CC BY-NC-SA
Details
Reduced mass of an  particle and a gold nucleus:
particle and a gold nucleus:

The constant  determines the sign and intensity of the electric force:
determines the sign and intensity of the electric force:

Initially the  particle must be sufficiently far from the nucleus; only the kinetic energy
particle must be sufficiently far from the nucleus; only the kinetic energy  is taken into account:
is taken into account:

In order to get an initial distance 20 times the nuclear radius the initial position is taken as:


Snapshots
Permanent Citation