Vapor Pressure and Density of Alkali Metals

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
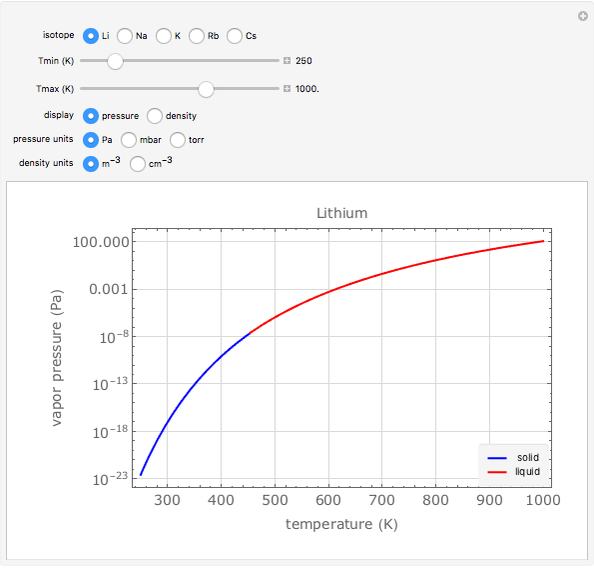
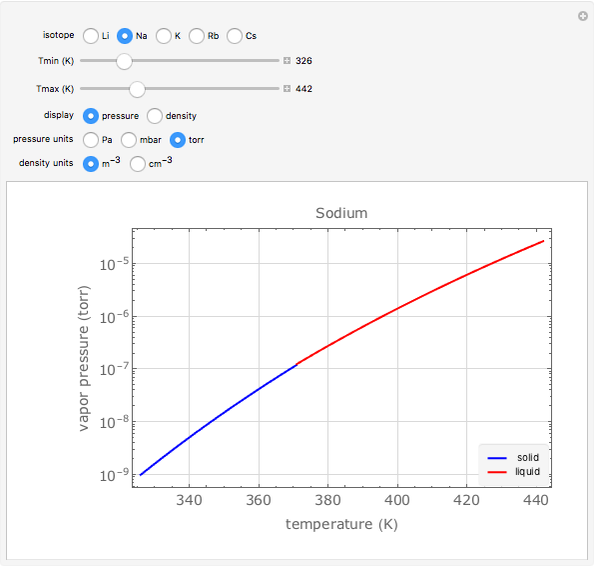
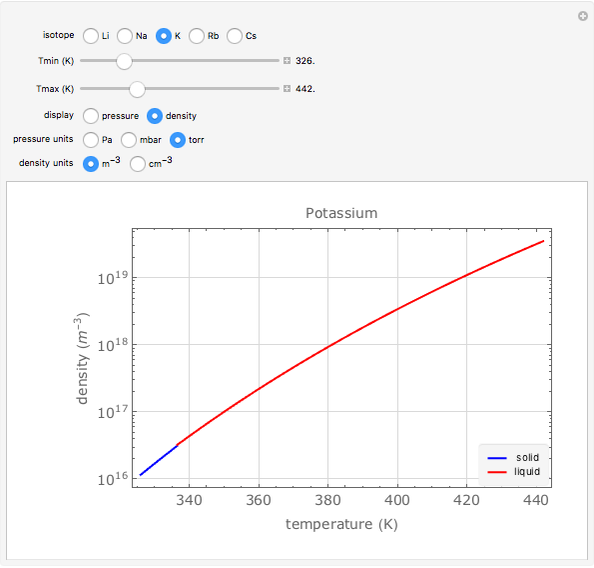
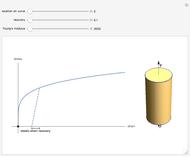
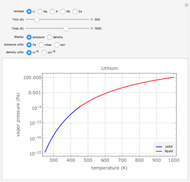
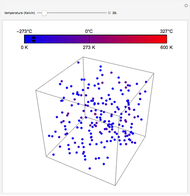
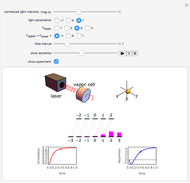
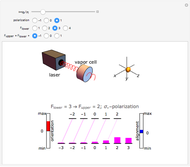
Plots of the temperature dependence of the vapor pressure and of the number density of vapors of alkali atoms in thermodynamic equilibrium with the liquid or solid metal.
Contributed by: Gianni Di Domenico (Université de Neuchâtel) and Antoine Weis (Université de Fribourg) (March 2011)
Open content licensed under CC BY-NC-SA
Snapshots
Details
When a liquid or solid material is in thermodynamic equilibrium with its vapor, the pressure  of the latter, called the vapor pressure, is a function of the temperature
of the latter, called the vapor pressure, is a function of the temperature  of the liquid/solid-vapor interface. In its simplest form the pressure
of the liquid/solid-vapor interface. In its simplest form the pressure  is given by the Clausius-Clapeyron law
is given by the Clausius-Clapeyron law
 , (1)
, (1)
where  is the latent heat of fusion (for solids), or the latent heat of vaporization (for liquids),
is the latent heat of fusion (for solids), or the latent heat of vaporization (for liquids),  the Boltzmann constant, and
the Boltzmann constant, and  the absolute temperature, measured in
the absolute temperature, measured in  .
.
Equation (1) can be rewritten as
 ,
,
where  and
and  are material- (and phase-) dependent constants. The constants
are material- (and phase-) dependent constants. The constants  and
and  used in this Demonstration are taken from an article by C. B. Alcock, V. P. Itkin, and M. K. Horrigan. They reproduce the observed pressures, reported in the 2003 CRC Handbook, to an accuracy of
used in this Demonstration are taken from an article by C. B. Alcock, V. P. Itkin, and M. K. Horrigan. They reproduce the observed pressures, reported in the 2003 CRC Handbook, to an accuracy of  or better (see the Wikipedia vapor pressure data page).
or better (see the Wikipedia vapor pressure data page).
The number density  of the vapor is given by
of the vapor is given by
 .
.
References:
C. B. Alcock, V. P. Itkin, and M. K. Horrigan, "Vapor Pressure of the Metallic Elements," Canadian Metallurgical Quarterly, 23, 1984 pp. 309–313.
D. Lide, ed., CRC Handbook of Chemistry and Physics, 84th ed., Boca Raton, FL: CRC, 2003.
Permanent Citation