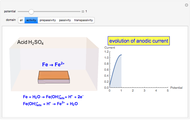
Electrode-Solution Interface: Distribution of Ions around an Electrode

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
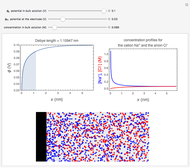
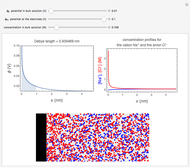
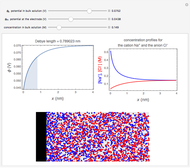
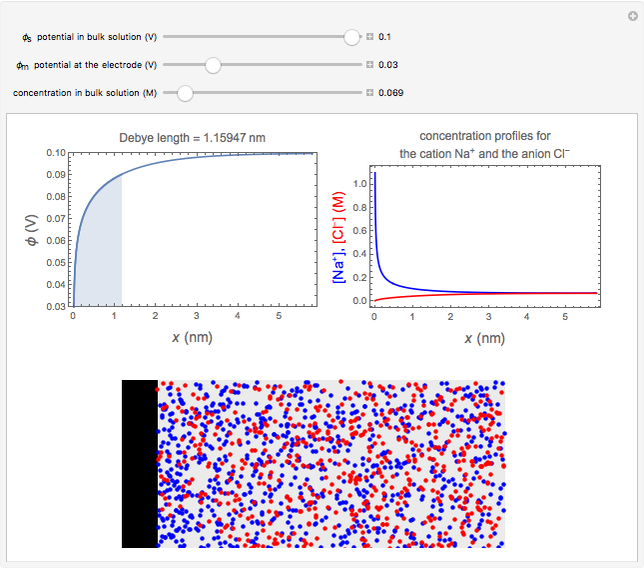
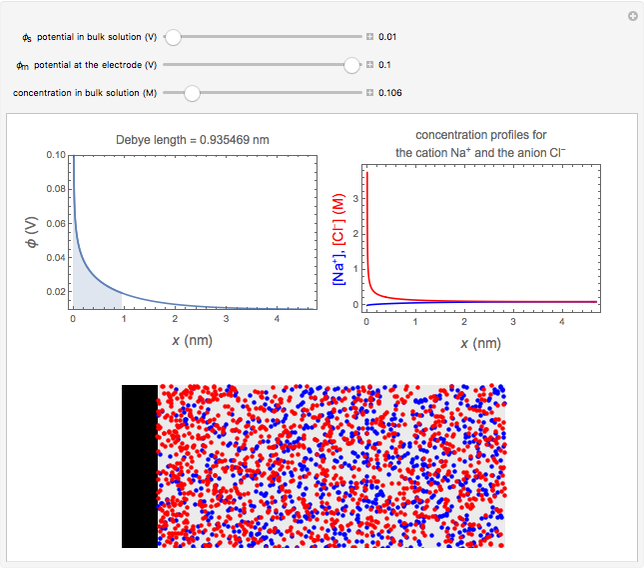
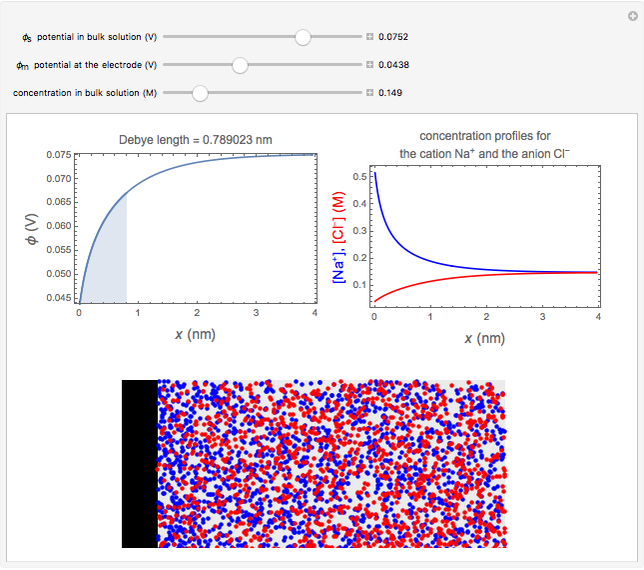
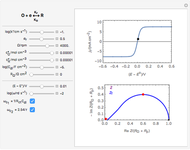
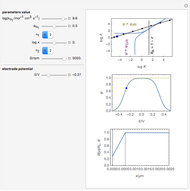
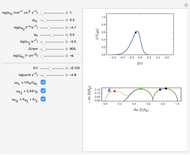
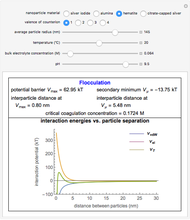
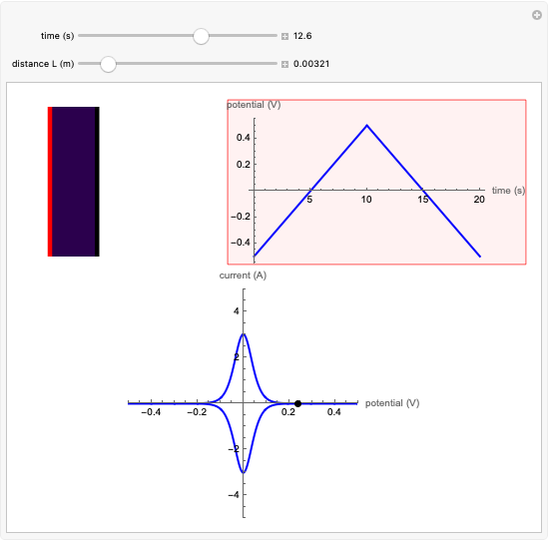
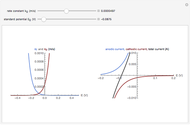
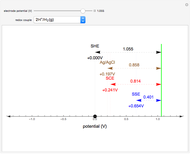
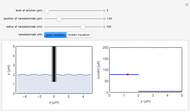
What happens if a bar of a noble metal is immersed in an electrolyte solution such as 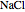 ? Due to the difference between the electric potential of the metal and the electrode-solution interface, the ions will move to equalize the total charge. This Demonstration shows the distribution of ions (cation
? Due to the difference between the electric potential of the metal and the electrode-solution interface, the ions will move to equalize the total charge. This Demonstration shows the distribution of ions (cation  and anion
and anion 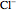 ) near an electrode surface and also the variation of potential with distance from the electrode, as functions of concentration.
) near an electrode surface and also the variation of potential with distance from the electrode, as functions of concentration.
Contributed by: Quang-Dao Trinh (July 2012)
Open content licensed under CC BY-NC-SA
Snapshots
Details
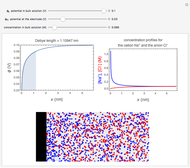
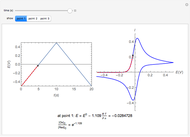
The distribution of ions is based on the Gouy–Chapman theory. The concentration profiles of  and
and 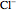 are colored blue and red, respectively. The electrode is positioned at
are colored blue and red, respectively. The electrode is positioned at  . The filled part on the potential diagram represents the Debye length (a characteristic distance at which significant charge separation can occur). The red and blue points represent
. The filled part on the potential diagram represents the Debye length (a characteristic distance at which significant charge separation can occur). The red and blue points represent 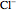 and
and  ions, respectively.
ions, respectively.
Permanent Citation