Oxidation Number of Carbon

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
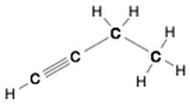
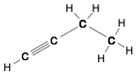
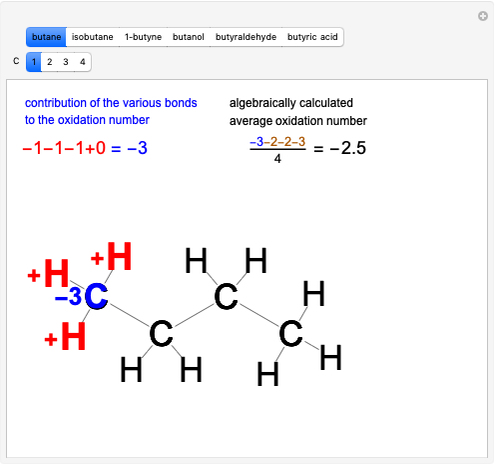
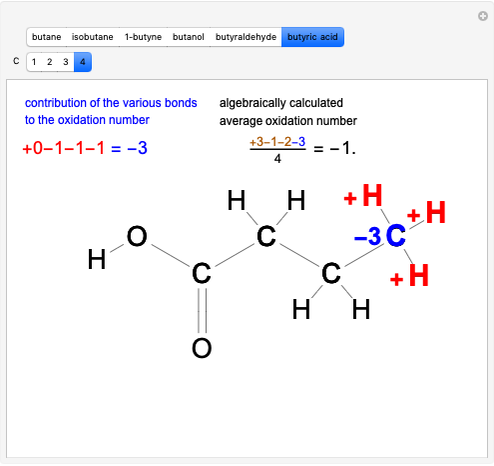
This Demonstration shows two ways of assigning an oxidation number to a carbon atom in an organic compound: algebraic and structural. Using the structure method [1], a formal charge is assumed, assigning the bonding electrons to the most electronegative atom. Bonding between atoms of the same species produces no formal charge difference. The same principle is applied with multiple bonds. Six different molecules are shown: butane, isobutane, 1-butyne, butanol, butyraldeide and butyric acid [2].
Contributed by: D. Meliga, A. Ratti, L. Lavagnino and S. Z. Lavagnino (June 2021)
Open content licensed under CC BY-NC-SA
Snapshots
Details
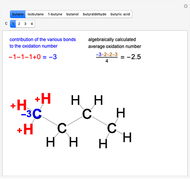
Snapshot 1: oxidation number of the carbon with an 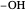 group
group
Snapshot 2: oxidation number of the carbon present in the 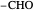 aldehydic group, obtained by little oxidation of alcohol
aldehydic group, obtained by little oxidation of alcohol
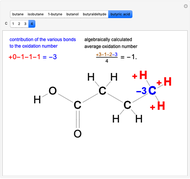
Snapshot 3: oxidation number of the carbon present in the 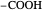 acid group, obtained by large oxidation of alcohol
acid group, obtained by large oxidation of alcohol
References
[1] S. Z. Lavagnino. Bilanciamento ossido riduzioni [Video]. (Jun 11, 2021) www.youtube.com/watch?v=U6oq6unRZMghttps://www.youtube.com/watch?v=U6oq6unRZMgNonehttps://www.youtube.com/watch?v=U6oq6unRZMgHyperlinkActionRecycledHyperlinkActiveHyperlinkRecycledHyperlinkTemplate.
[2] H. Hart, L. E. Craine, D. J. Hart and W. W. Linstromberg, Organic Chemistry: A Short Course, 10th ed., Boston: Houghton Mifflin, Co., 1999.
Permanent Citation