Pauling Electronegativity and Dipole Moment

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
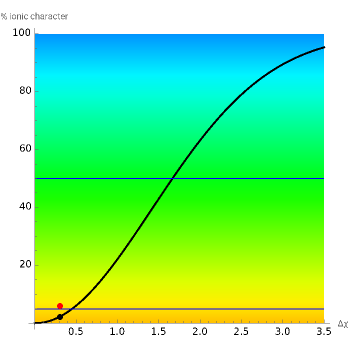
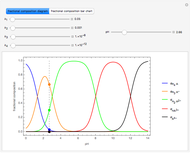
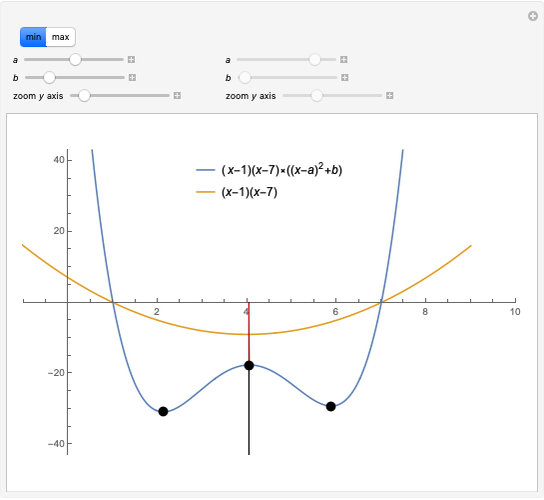
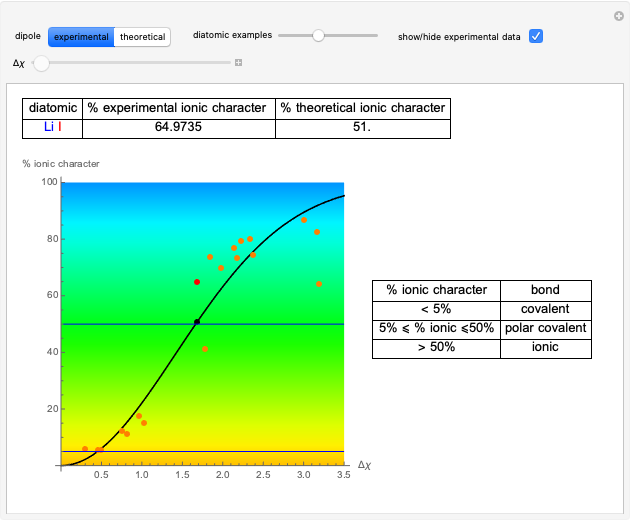
This Demonstration shows the relationship between the dipole moment of molecules and Pauling's electronegativity. Pauling defined electronegativity [1] and proposed the following relation:  . This is an empirical approximation and sometimes is not accurate. More recent approaches are more complex and depend on other electronic properties of the molecule [2].
. This is an empirical approximation and sometimes is not accurate. More recent approaches are more complex and depend on other electronic properties of the molecule [2].
Contributed by: D. Meliga and S. Z. Lavagnino (January 2020)
Open content licensed under CC BY-NC-SA
Snapshots
Details
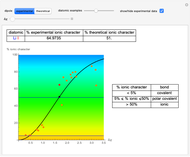
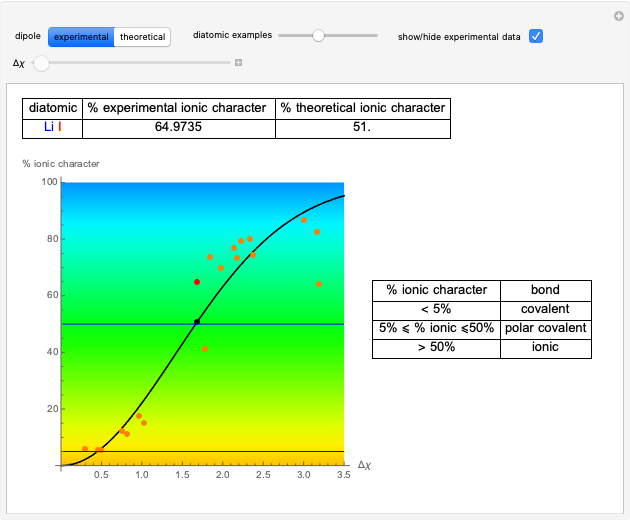
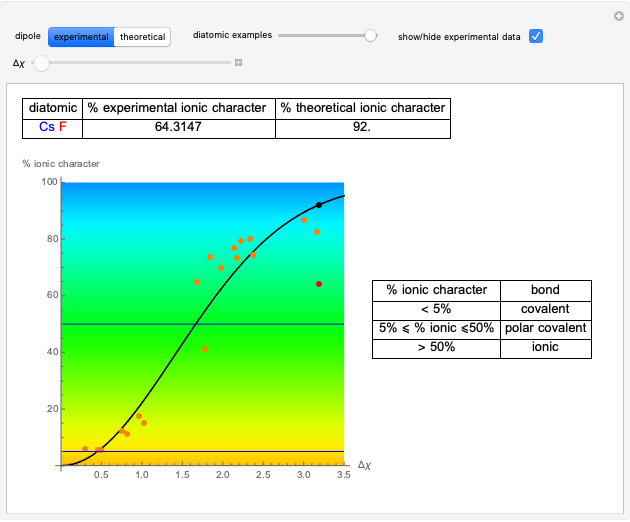
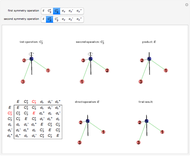
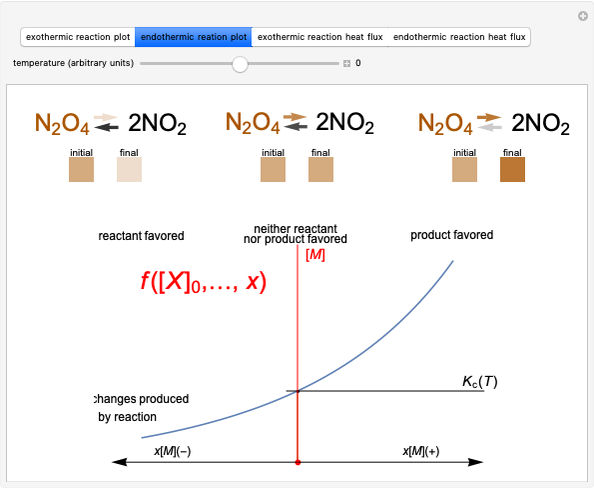
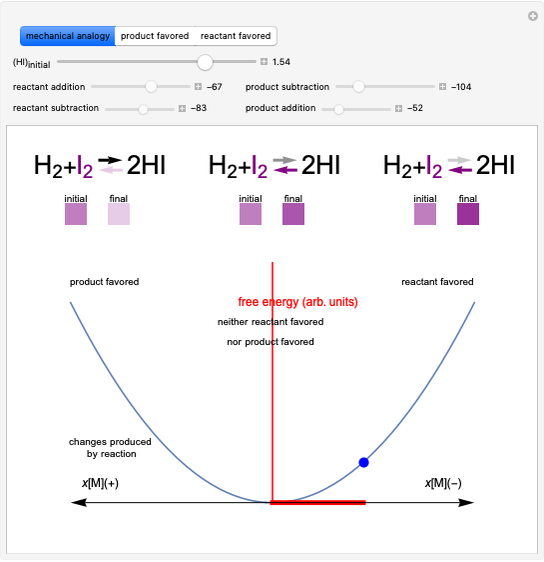
Snapshot 1: Discrepancy between experimental and theoretical percentage of ionic character of the CsF molecule. The predicted value is lower than the experimental one, but this is still classified as an ionic bond.
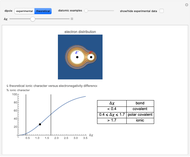
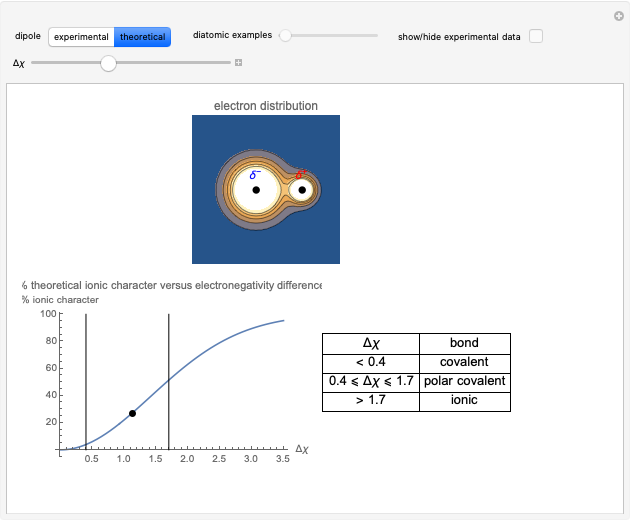
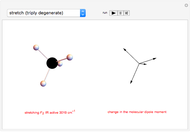
Snapshot 2: Theoretical model of a polar covalent bond. Bonding electrons are shared unequally between the atoms forming partial charges ( ) on the atoms.
) on the atoms.
Snapshot 3: Theoretical model of a ionic bond. Ions are formed as a consequence of the complete migration of one electron. The charge displacement is neat in this case.
References
[1] LibreTexts. "Electronegativity and Dipole Moment." (Jan 14, 2020) chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Map%3A_Physical_Chemistry_for_the_Biosciences_(Chang)/12%3A_The_Chemical_Bond/12.4%3A_Electronegativity_and_Dipole_Moment.
[2] Quora. "What Is the Relation of Ionic Character with Anionic Charge?" (Jan 14, 2020) www.quora.com/What-is-the-relation-of-ionic-character-with-anionic-charge.
[3] "9.3 Polarity of Molecules." (Jan 14, 2020) wps.prenhall.com/wps/media/objects/3081/3155729/blb0903.html.
[4] StackExchange. "Why Are Bonds Ionic when the Electronegativity Difference between Bonded Atoms Is Greater than 1.7?" (Jan 14, 2020) chemistry.stackexchange.com/questions/9222/why-are-bonds-ionic-when-the-electronegativity-difference-between-bonded-atoms-i.
Permanent Citation