Substituted Pyrimidines and Purines

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
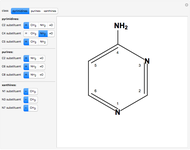
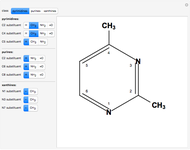
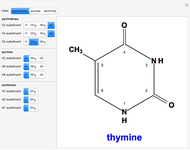
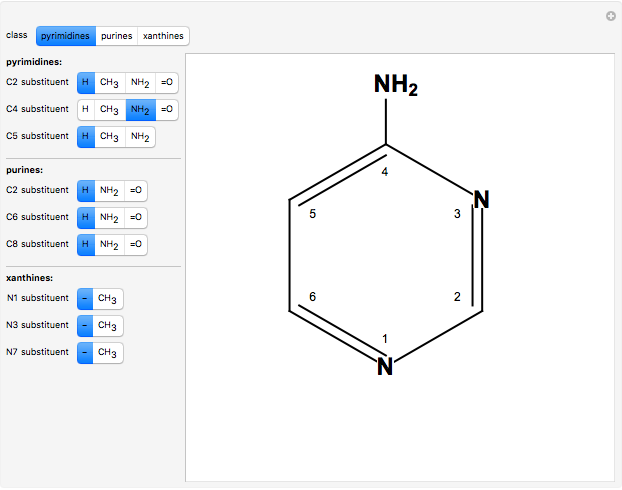
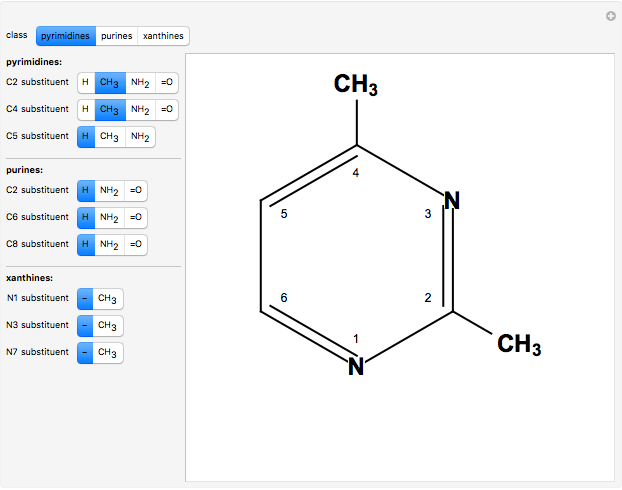
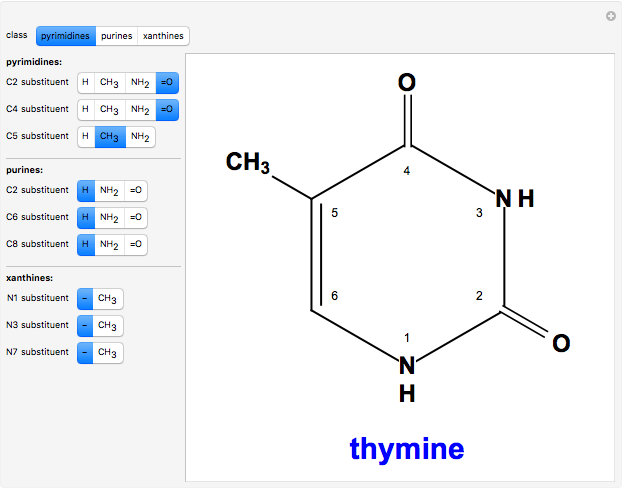
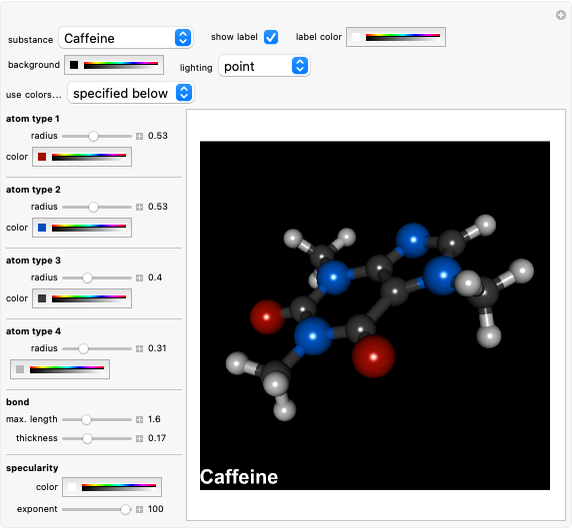
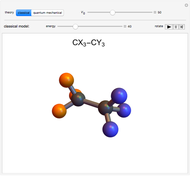
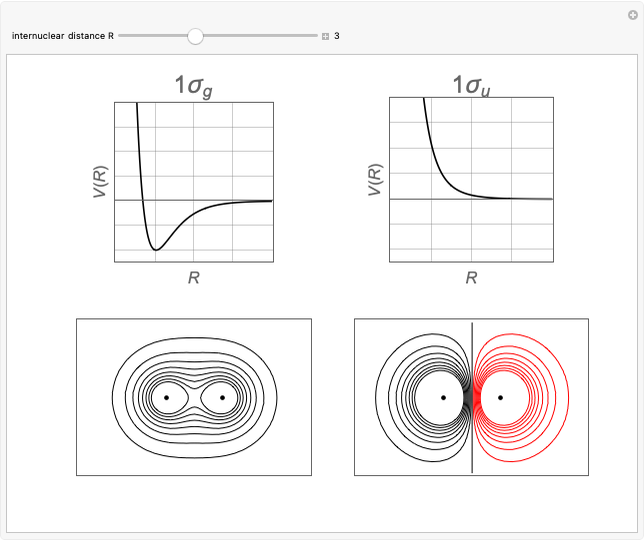
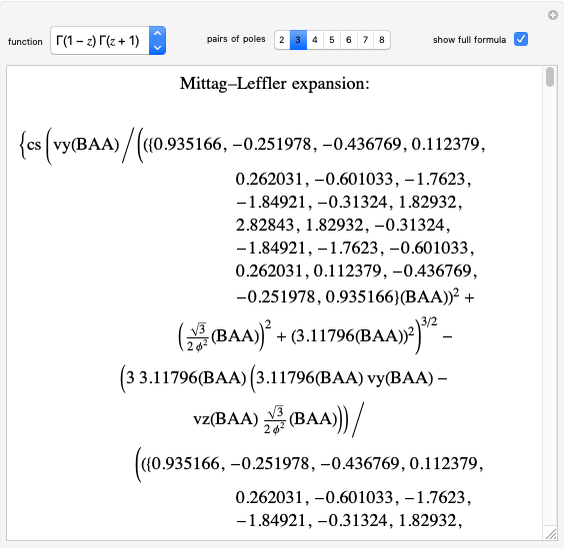
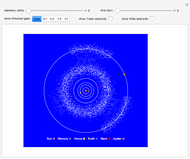
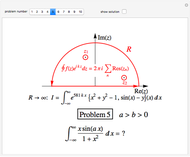
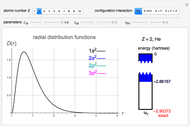
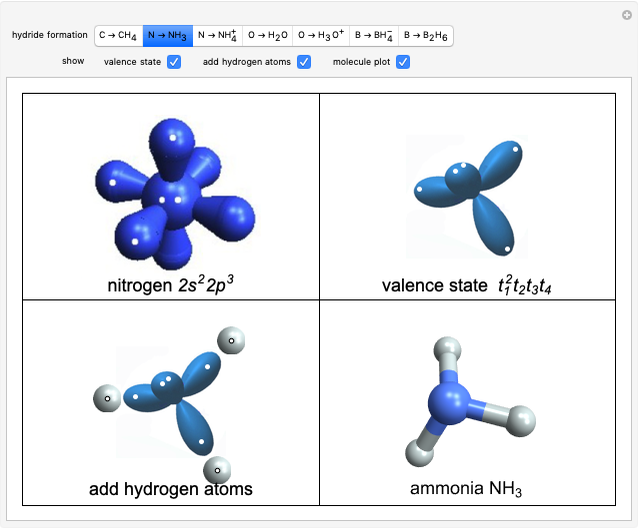
Two classes of nitrogen heterocycles, derivatives of pyrimidine and purine, occur in many biologically important compounds. This Demonstration allows you to substitute methyl (-C ), amide (-N
), amide (-N ), and oxy (=O) groups in various positions of each class of molecules. Although many of the possible outcomes have no special significance, you can create all the bases of DNA and RNA, as well as natural products such as uric acid, caffeine, and theobromine. The latter two belong to the category of substituted xanthines. Formal addition of an -OH group usually results in an enol-keto transformation, which is shown by shifting double bonds and hydrogen atoms in the structures. Standard nomenclature for substituted pyrimidines and purine is indicated in several examples in the Details section.
), and oxy (=O) groups in various positions of each class of molecules. Although many of the possible outcomes have no special significance, you can create all the bases of DNA and RNA, as well as natural products such as uric acid, caffeine, and theobromine. The latter two belong to the category of substituted xanthines. Formal addition of an -OH group usually results in an enol-keto transformation, which is shown by shifting double bonds and hydrogen atoms in the structures. Standard nomenclature for substituted pyrimidines and purine is indicated in several examples in the Details section.
Contributed by: S. M. Blinder (March 2011)
Open content licensed under CC BY-NC-SA
Snapshots
Details
Snapshot 1: 4-aminopyrimidine
Snapshot 2: 2,4-dimethylpyrimidine
Snapshot 3: 2,4-dioxy-5-methylpyrimidine (thymine)
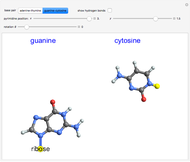
Snapshot 4: 2-oxy-4-aminopyrimidine (cytosine)
Snapshot 5: 6-methylpurine (adenine)
Snapshot 6: 2-amino-6-oxypurine (guanine)
Snapshot 7: 2,6,8-trioxypurine (uric acid)
Snapshot 8: 2,6-dioxypurine (xanthine)
Snapshot 9: 2,6-dioxy-3,7-dimethylpurine (theobromine, "food of the gods"), a component of chocolate
Wikipedia references: Pyrimidine, Purine, Xanthine
Permanent Citation
"Substituted Pyrimidines and Purines"
http://demonstrations.wolfram.com/SubstitutedPyrimidinesAndPurines/
Wolfram Demonstrations Project
Published: March 7 2011