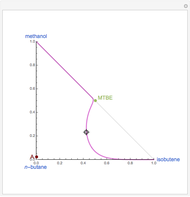
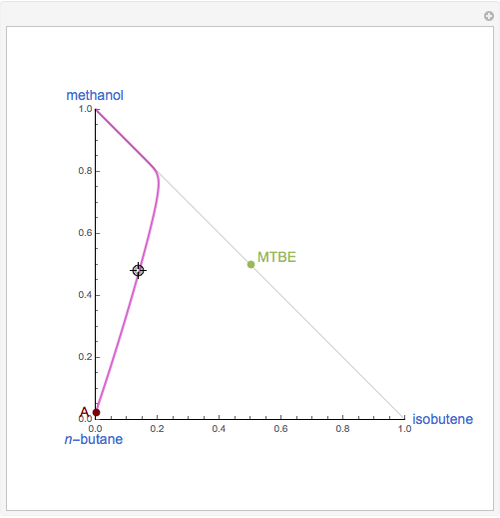
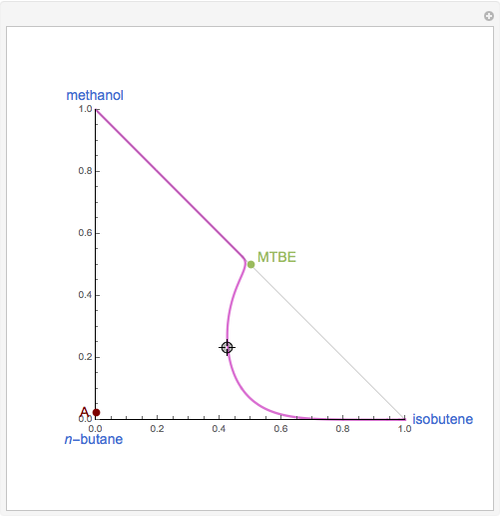
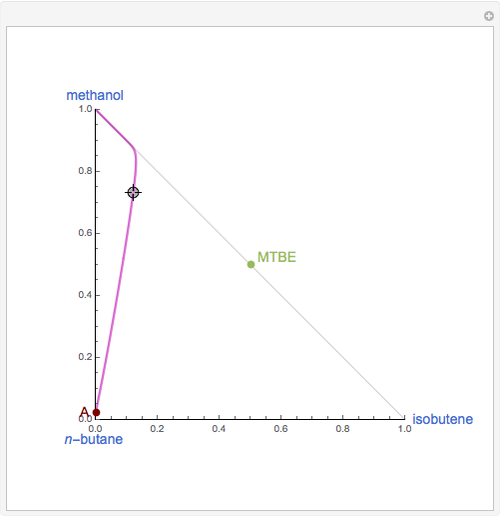
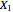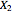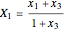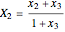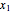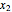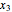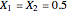Residue Curve Computation for Methyl Tert-Butyl Ether (MTBE) Chemistry
Initializing live version

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
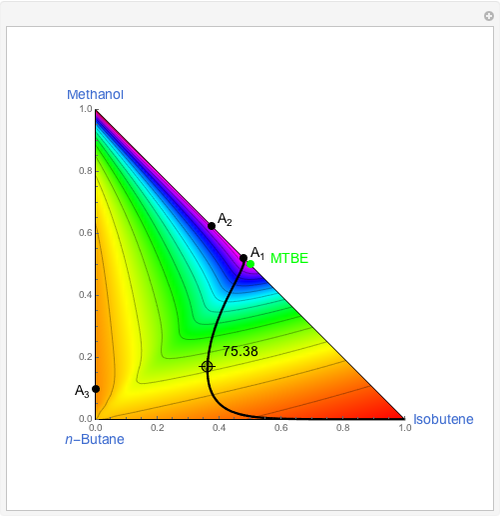
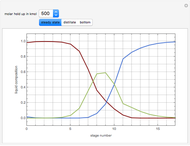
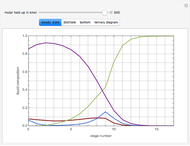
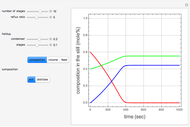
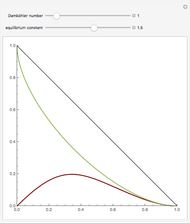
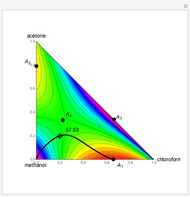
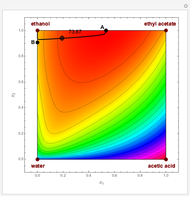
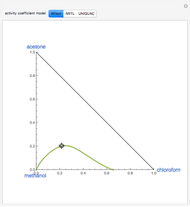
The following reaction is used to produce methyl tert-butyl ether (MTBE): isobutene+MeOH ⇄ MTBE. The reacting mixture contains  -butane, an inert component, in addition to methanol (MeOH), isobutene, and MTBE.
-butane, an inert component, in addition to methanol (MeOH), isobutene, and MTBE.
Contributed by: Housam Binous and Ikbel El Glaoui (March 2011)
Open content licensed under CC BY-NC-SA
Snapshots
Details
References:
Wikipedia, "Methyl Tert-Butyl Ether".
M. Niang and P. Mikitenko, "Analyse de Faisabilité d'une Distillation Réactive par la Méthode des Courbes de Résidus," Revue de l'Institut Français du Pétrole, 53(4), 1998 pp. 439–462.
Permanent Citation