Isobaric Vapor Liquid Equilibrium Computations

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
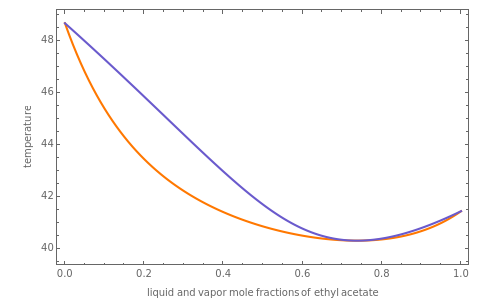
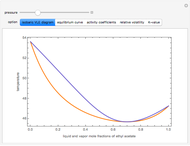
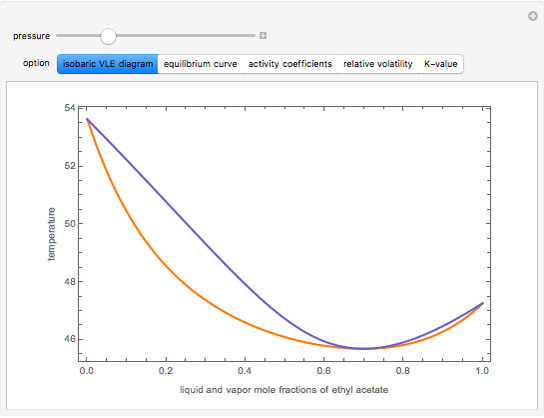
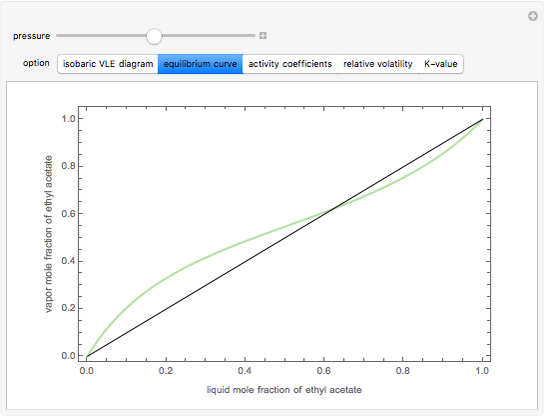
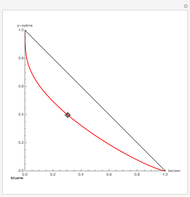
This Demonstration computes several vapor-liquid properties of the ethanol-ethyl acetate binary mixture, such as the isobaric vapor liquid equilibrium (VLE) diagram  ), the equilibrium curve (
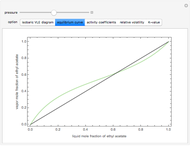
), the equilibrium curve ( ), the relative volatility (
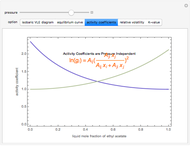
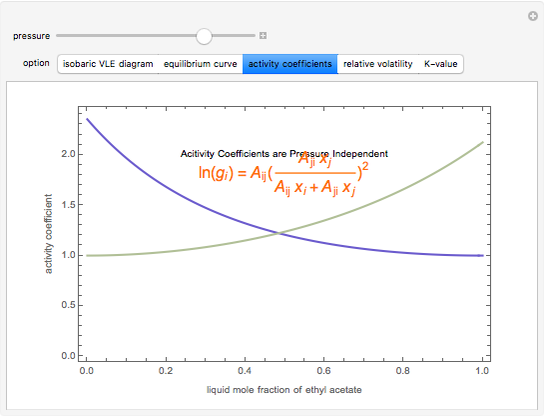
), the relative volatility ( ), the activity coefficients (
), the activity coefficients ( ), and the K-value (
), and the K-value ( ). You can vary the pressure up to atmospheric pressure (760 mm Hg). Vapor phase is assumed to be ideal at such low pressures. Deviation from ideality in the liquid phase is taken into account using the Van Laar model, which allows the computation of the activity coefficients. Antoine's equation is used to model saturated vapor pressure's dependence on temperature.
). You can vary the pressure up to atmospheric pressure (760 mm Hg). Vapor phase is assumed to be ideal at such low pressures. Deviation from ideality in the liquid phase is taken into account using the Van Laar model, which allows the computation of the activity coefficients. Antoine's equation is used to model saturated vapor pressure's dependence on temperature.
Contributed by: Housam Binous (March 2011)
Open content licensed under CC BY-NC-SA
Snapshots
Details
detailSectionParagraphPermanent Citation
"Isobaric Vapor Liquid Equilibrium Computations"
http://demonstrations.wolfram.com/IsobaricVaporLiquidEquilibriumComputations/
Wolfram Demonstrations Project
Published: March 7 2011