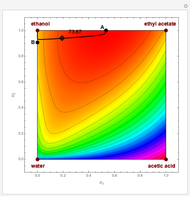
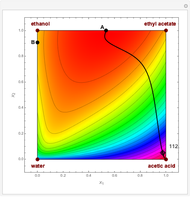
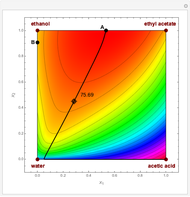
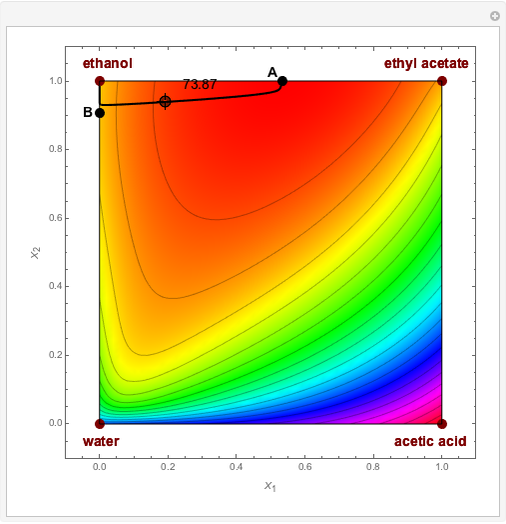
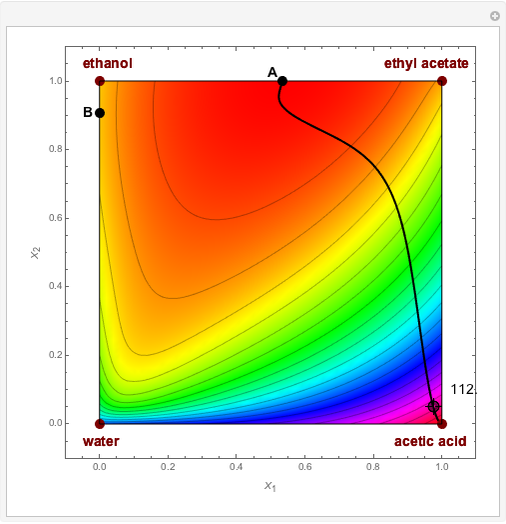
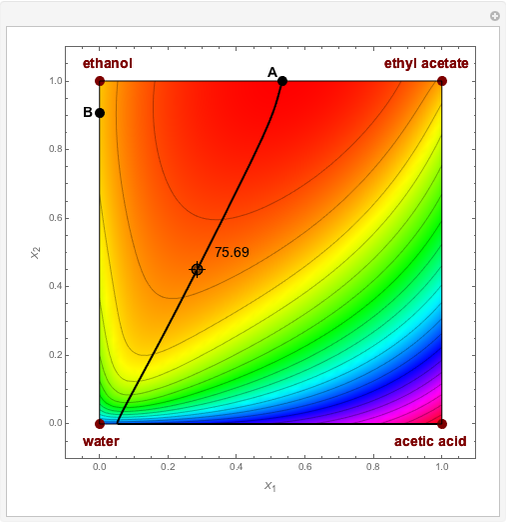
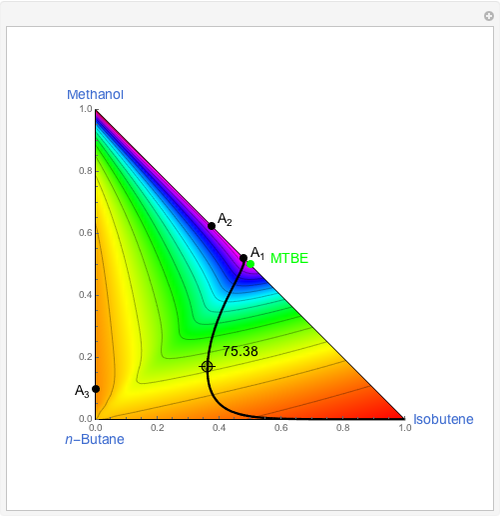
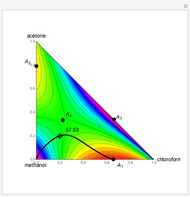
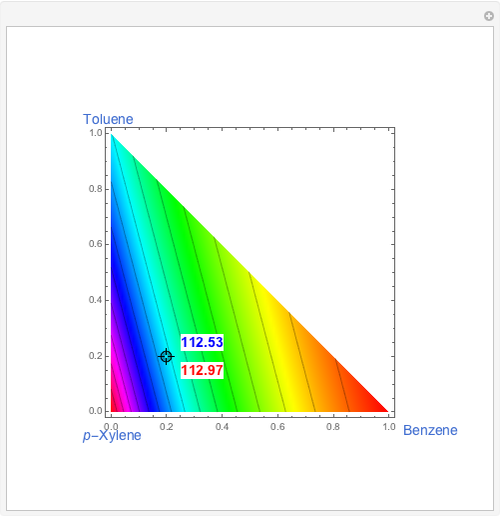
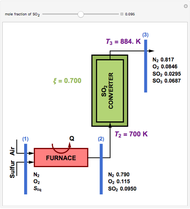
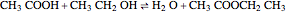Residue Curve and Temperature Distribution for Ethyl Acetate Chemistry
Initializing live version

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
The esterification reaction between ethanol and acetic acid produces water and ethyl acetate:
[more]
Contributed by: Housam Binous, Ikbel El Glaoui, and Ahmed Bellagi (March 2011)
Open content licensed under CC BY-NC-SA
Snapshots
Details
For more information, see:
M. F. Doherty and M. F. Malone, Conceptual Design of Distillation Systems, New York: McGraw-Hill, 2001.
Permanent Citation