Ohmic Drop and Uncompensated Electrolyte Resistance in an Electrochemical Cell

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
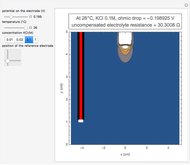
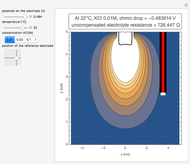
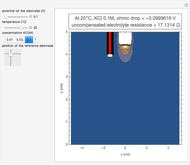
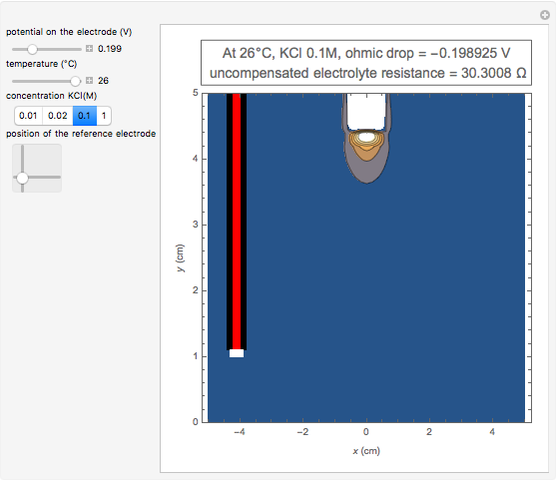
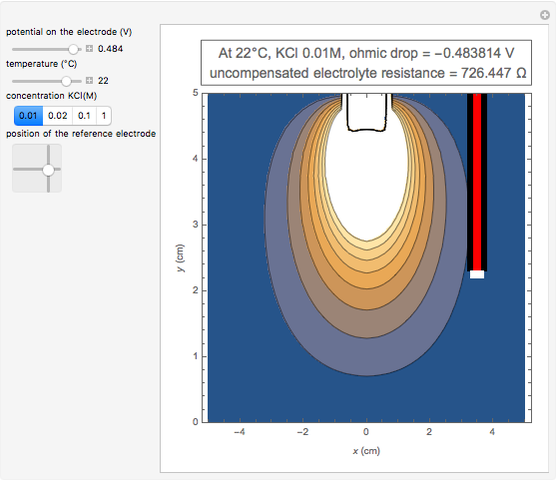
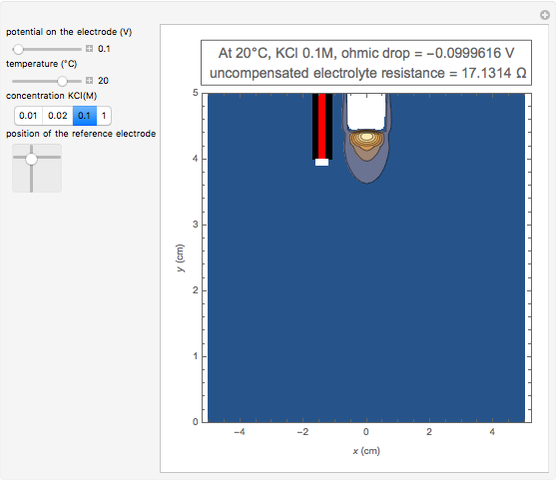
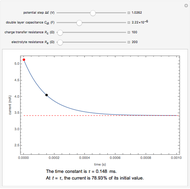
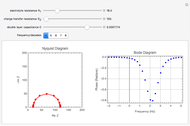
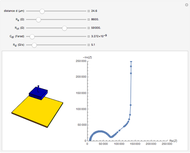
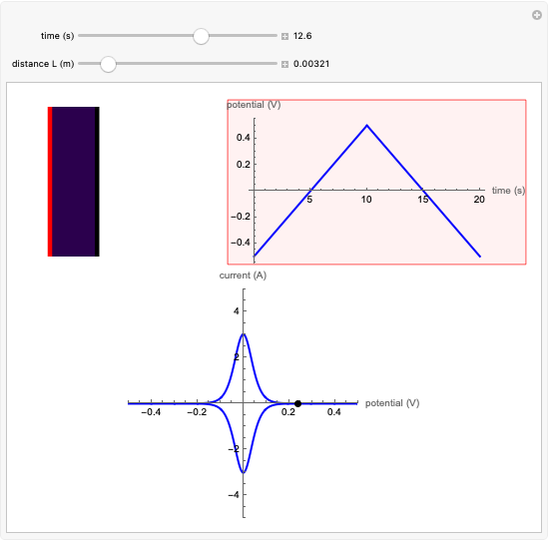
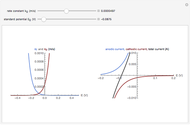
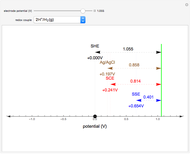
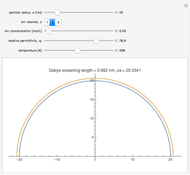
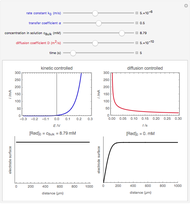
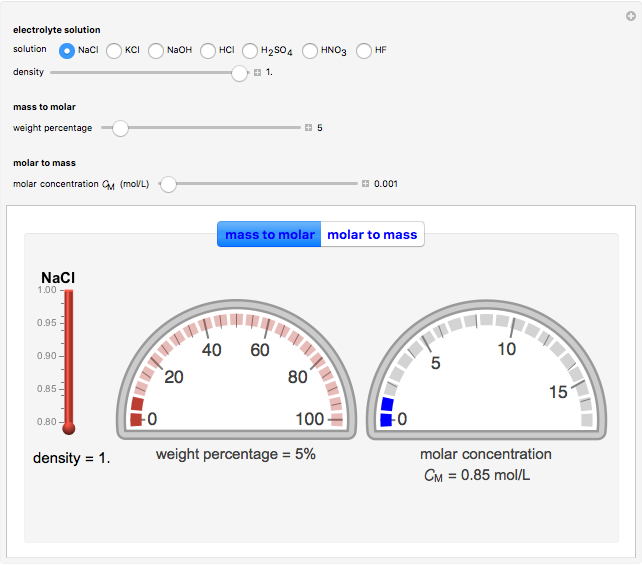
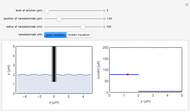
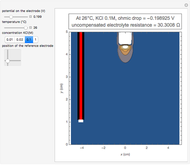
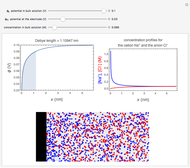
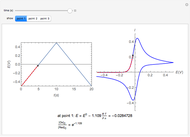
In an electrochemical cell, the ohmic potential drop in the bulk of the solution cannot be negligible. This Demonstration shows the dependence of the ohmic drop and the resistance electrolyte on temperature, concentration of the supporting electrolyte (0.25 cm of radius), and position of the reference electrode (saturated calomel electrode). By changing the position of the reference electrode in the cell, you can vary the ohmic potential drop between the working disk electrode and the reference electrode. With the higher concentration of the supporting electrolyte, the resistance electrolyte decreases. The ambient temperature can also slightly affect the ohmic potential drop.
Contributed by: Quang-Dao Trinh (August 2012)
Open content licensed under CC BY-NC-SA