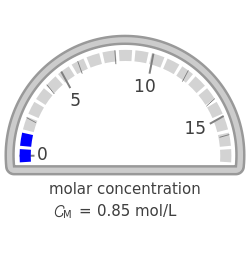
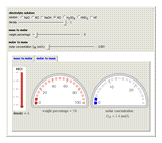
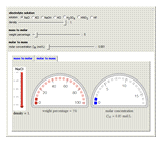
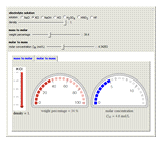
Molar and Mass Concentration Conversion for Electrolytes

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
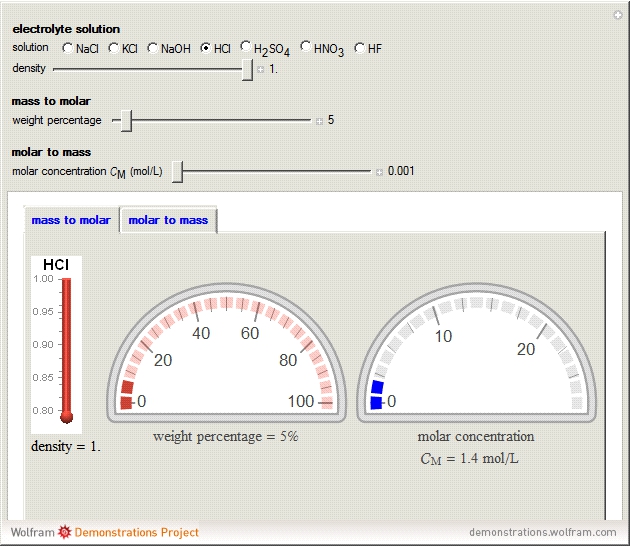
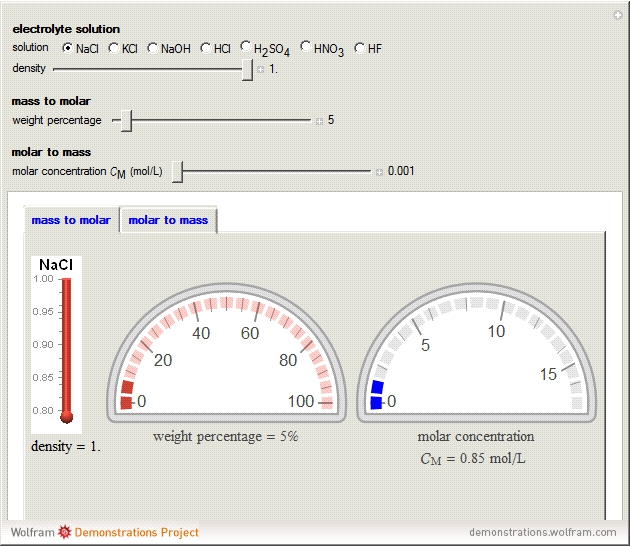
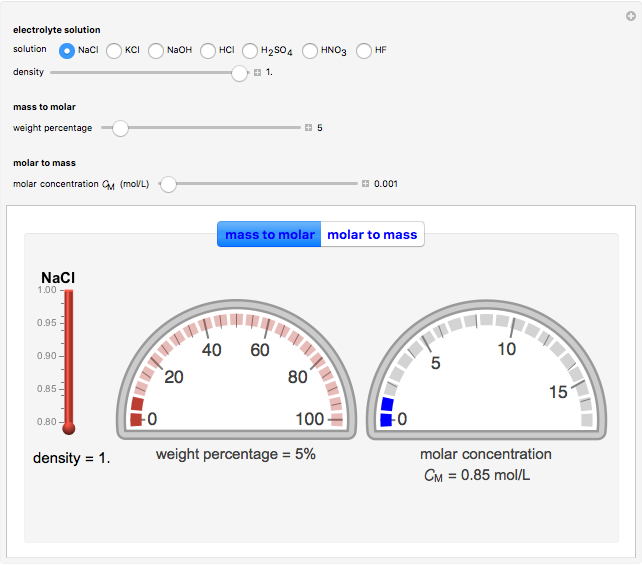
During the preparation of the electrolytes in electrochemistry, you can use either the molar concentration or the mass concentration (also designated wt%). This Demonstration will enable you to quickly convert between the molar concentration and the mass concentration. For example, you can quickly estimate the molar concentration of a 3 wt% NaCl solution or the mass concentration of a 0.5 M solution.
Contributed by: Quang-Dao Trinh (March 2016)
Open content licensed under CC BY-NC-SA
Snapshots
Details
Choose the electrolytes from the list, as well as the solution concentration. You can slide the concentration value or choose the value from the list. The corresponding concentration is calculated from the molar mass and the density of the solution. The density (or, more accurately, specific gravity) of an aqueous solution is approximately equal to 1. If you have specific data for a given solution, you can input this using the "density" slider.
Permanent Citation
"Molar and Mass Concentration Conversion for Electrolytes"
http://demonstrations.wolfram.com/MolarAndMassConcentrationConversionForElectrolytes/
Wolfram Demonstrations Project
Published: March 22 2016