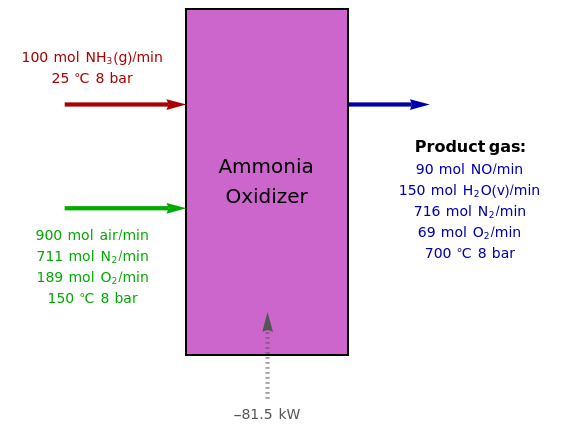
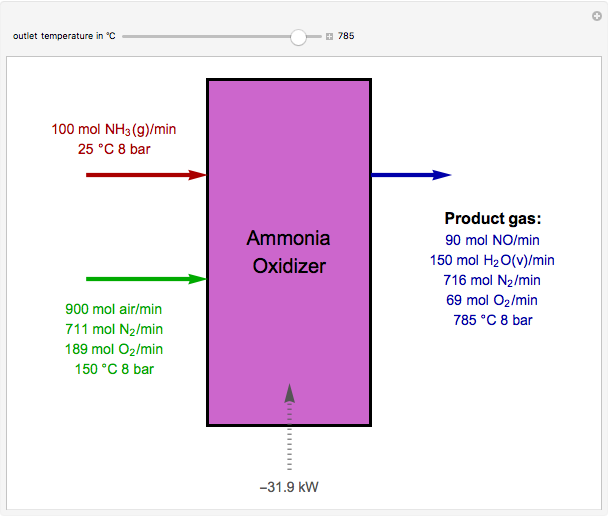
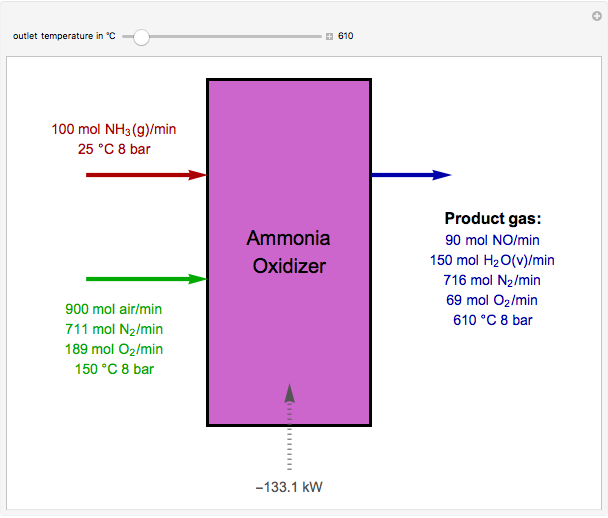
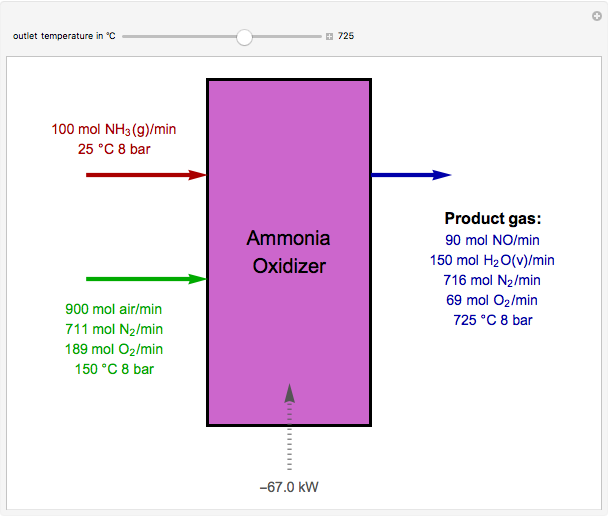
Oxidation of Ammonia by Air
Initializing live version

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
The standard enthalpies of reactions R1 and R2 are  and
and  , respectively. All of the heat requirements for the formations are obtained from [1, Appendix B1].
, respectively. All of the heat requirements for the formations are obtained from [1, Appendix B1].
Contributed by: Housam Binous and Ahmed Bellagi (September 2016)
Open content licensed under CC BY-NC-SA
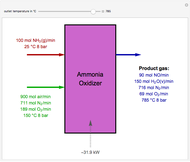
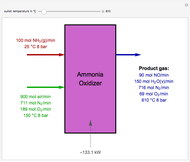
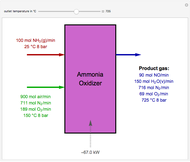
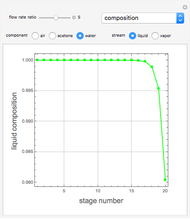
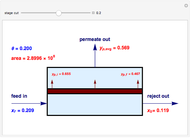
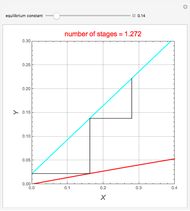
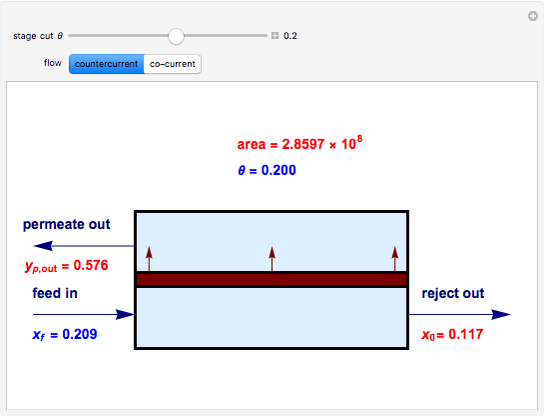
Snapshots
Details
Reference
[1] R. M. Felder and R. W. Rousseau, Elementary Principles of Chemical Processes, 3rd ed., New York: John Wiley & Sons, 2004.
Permanent Citation