Eliminating Acetone in Air Using Absorption by Water

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
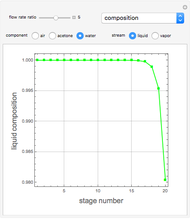
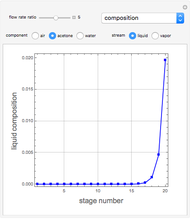
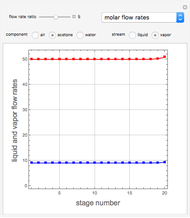
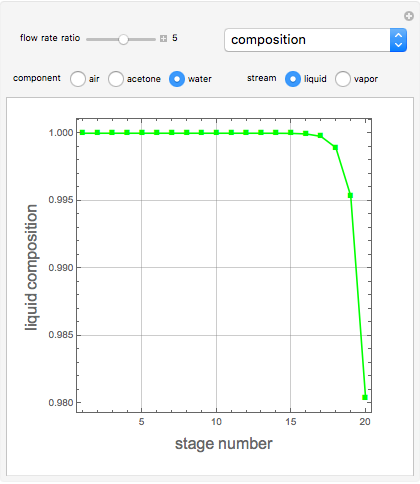
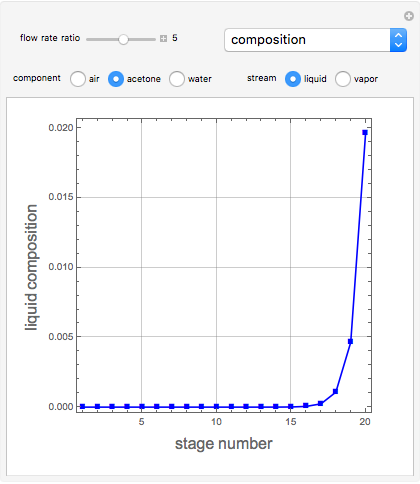
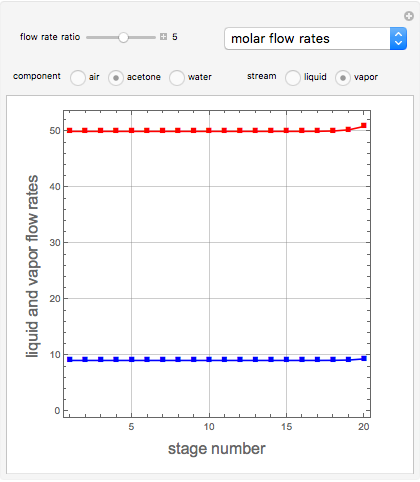
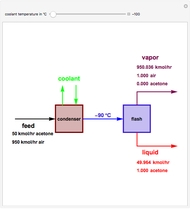
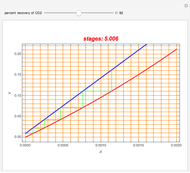
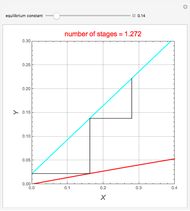
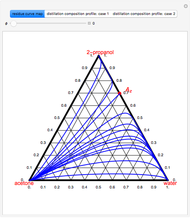
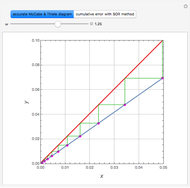
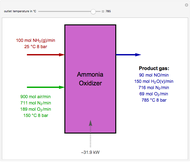
Consider a vapor stream composed of 90 mole% air and 10 mole% acetone. This stream is fed into an absorption column operating at 300 kPa and which has 20 stages. The solvent used to remove acetone from air is pure water. You can change the solvent to feed flow-rate ratio. This Demonstration calculates the vapor and liquid composition profiles inside the column as well as the temperature and molar flow rates. The calculation is rigorous since it solves the full MESH (mass, equilibrium, summation, and enthalpy) equation. It is possible to obtain a percent recovery of acetone equal to 100% since acetone is readily soluble in water. Comparison with Aspen HYSYS shows excellent agreement (HYSYS and Mathematica results are indicated by the open circles and solid squares, respectively).
Contributed by: Housam Binous and Ahmed Bellagi (December 2012)
Open content licensed under CC BY-NC-SA
Snapshots
Details
Expressions for pure component molar vapor and liquid enthalpies were adapted from Aspen HYSYS.
The mixture is assumed to obey the modified Raoult's law with Antoine constants taken from Aspen HYSYS and activity coefficients predicted using the Wilson model [2].
References
[1] E. J. Henley and J. D. Seader, Equilibrium-Stage Separation Operations in Chemical Engineering, New York: Wiley, 1981.
[2] G. M. Wilson, "Vapor-Liquid Equilibrium XI: A New Expression for the Excess Free Energy of Mixing," Journal of the American Chemical Society, 86(2), 1964 pp. 127–130. doi:10.1021/ja01056a002.
Permanent Citation
"Eliminating Acetone in Air Using Absorption by Water"
http://demonstrations.wolfram.com/EliminatingAcetoneInAirUsingAbsorptionByWater/
Wolfram Demonstrations Project
Published: December 18 2012