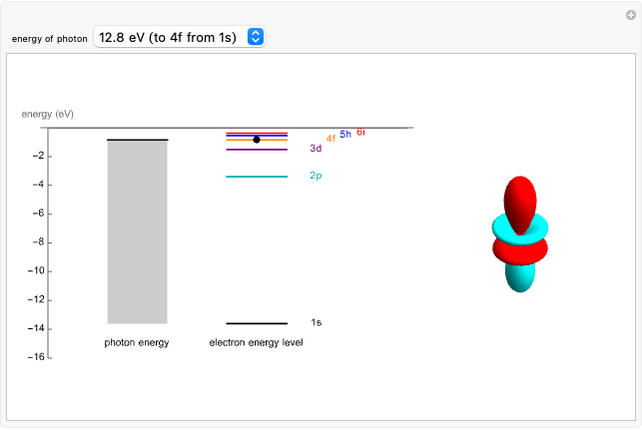
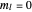Radiative Transitions in a Hydrogen Atom
Initializing live version

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
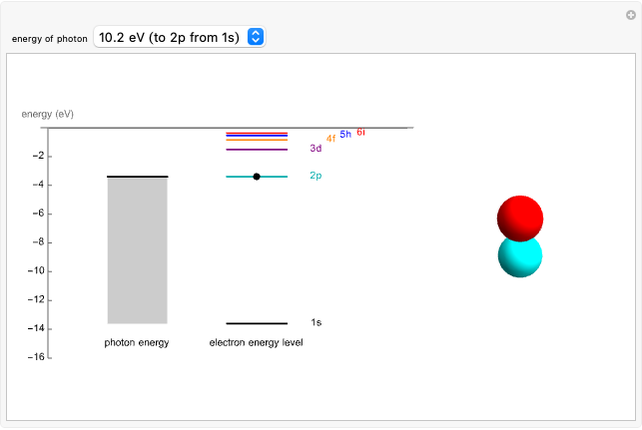
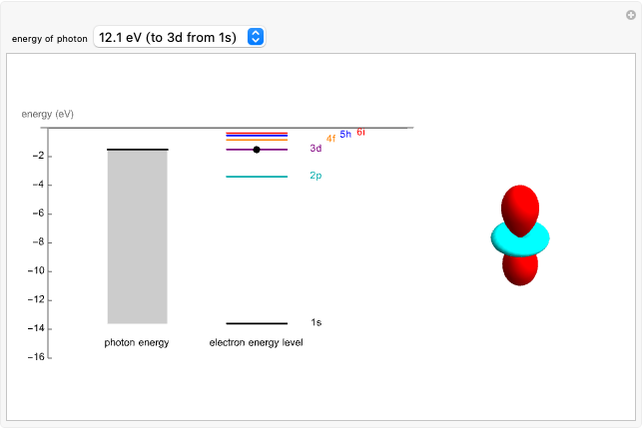
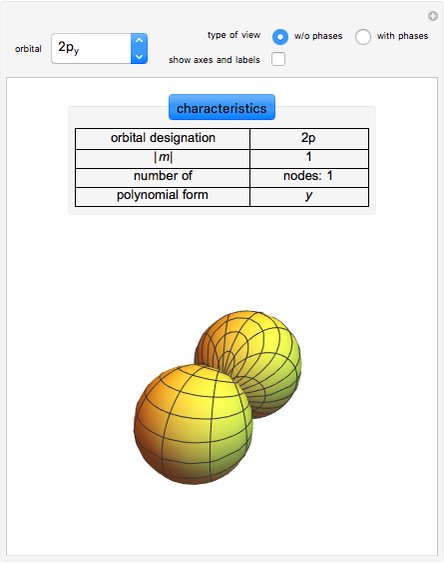
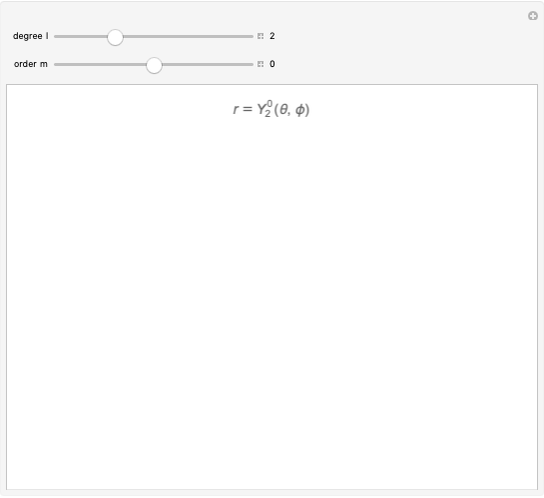
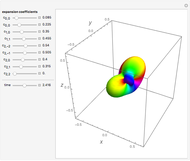
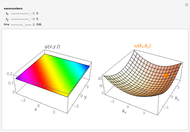
An electron in an atom or molecule can absorb energy from a photon and be excited to a higher energy level. This Demonstration considers a photon interacting with an electron in a hydrogen atom. A photon is absorbed only if its energy  is equal to an energy-level difference such that
is equal to an energy-level difference such that 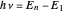 .
.
Contributed by: Mike Li and Luke Goodhope (August 27)
With additional contributions by: Heidi Hendrickson
Based on a program by: Porscha McRobbie and Eitan Geva
Open content licensed under CC BY-NC-SA
Details
Snapshots
Permanent Citation