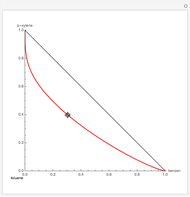
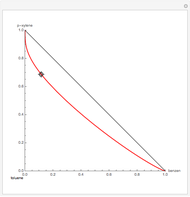
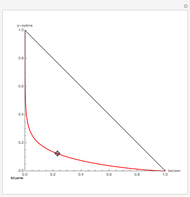
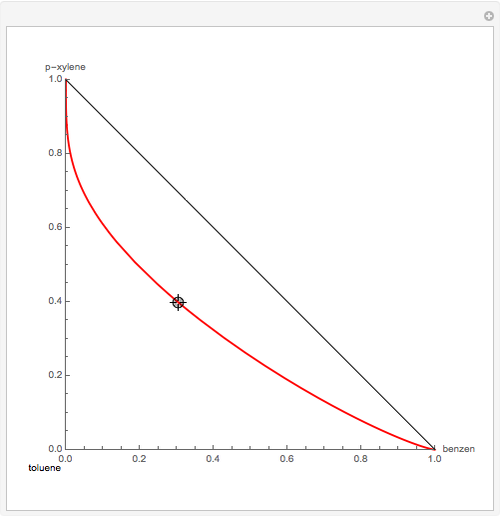
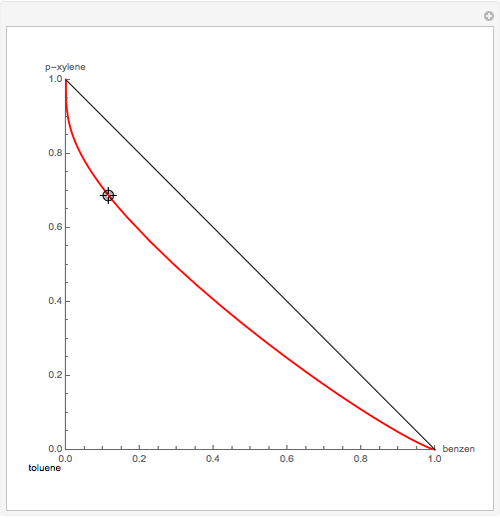
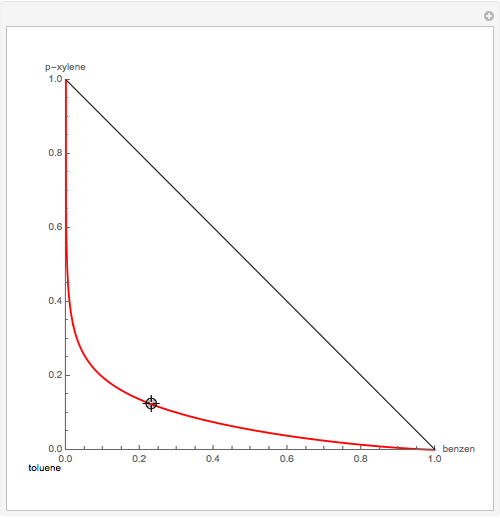
Residue Curve Map for a Benzene-Toluene-p-Xylene Mixture
Initializing live version

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
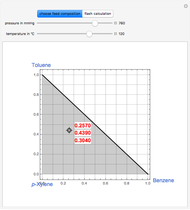
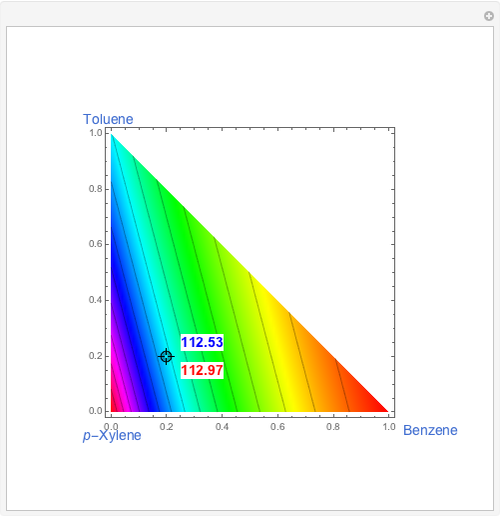
The Demonstration computes the residue curve map for the ternary mixture benzene-toluene-p-xylene. When you change the locator's position, a new residue curve is computed and displayed. Residue curve maps play an important role in the conceptual design of distillation columns.
[more]
Contributed by: Housam Binous (March 2011)
Open content licensed under CC BY-NC-SA
Snapshots
Details
For more information, see:
M. F. Doherty and M. F. Malone, Conceptual Design of Distillation Systems, New York: McGraw-Hill, 2001.
Permanent Citation