Salt Packs for Heating or Cooling

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
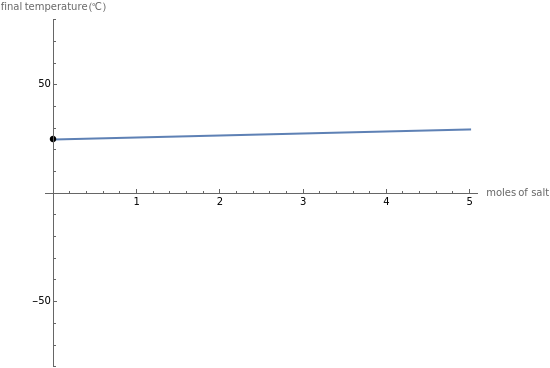
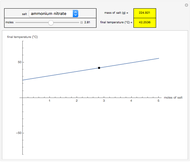
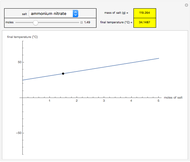
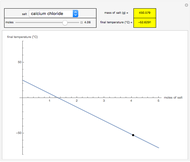
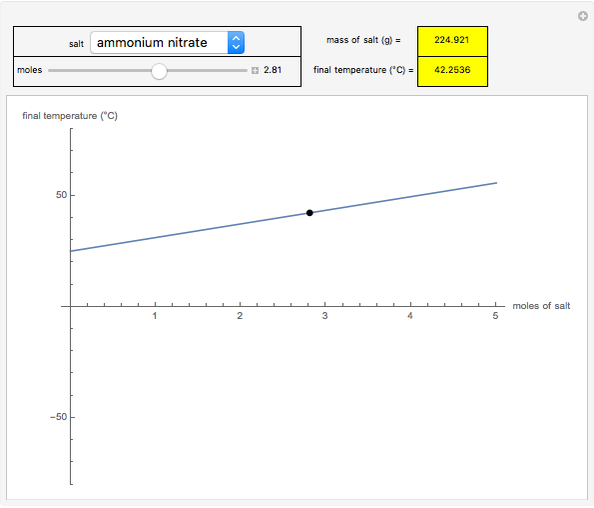
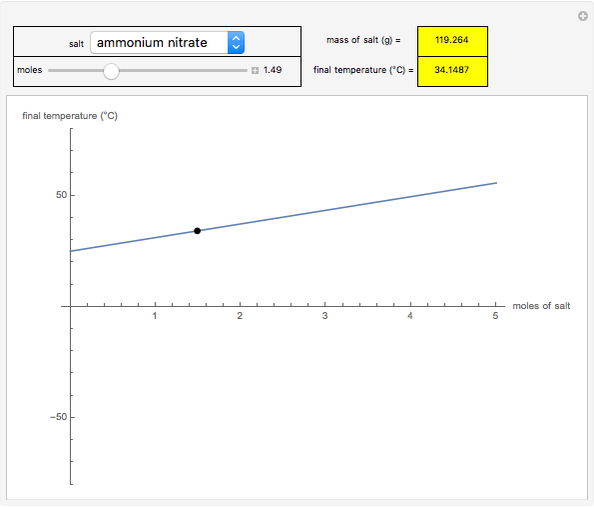
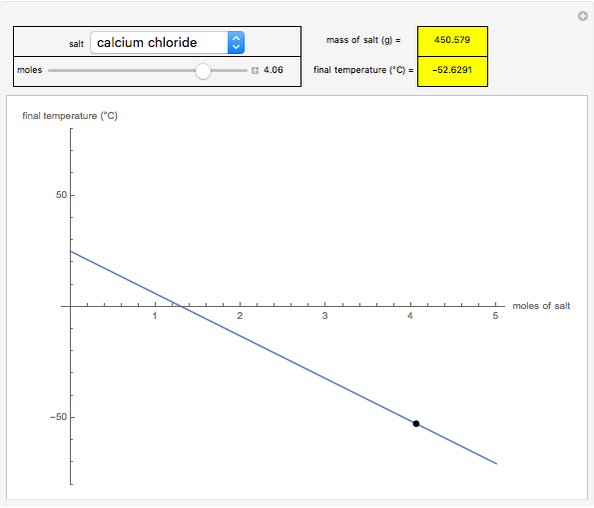
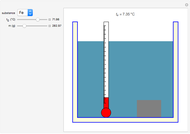
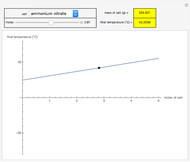
This Demonstration shows the heating or cooling effects achieved by salt packs. The chemical processes involved in producing efficient heat and cold packs depend on the enthalpy of dissolution of the salts and the specific heat of water. We investigate the theoretical effect of adding various amounts of salt to one kilogram of water at an initial temperature of 25 °C. The relevant formulas are  and
and  mol, where
mol, where  is the heat transferred (in J),
is the heat transferred (in J),  is the mass of salt (in g),
is the mass of salt (in g),  is the specific heat (in J/g K),
is the specific heat (in J/g K),  is the enthalpy change in the solute (J/mol) and
is the enthalpy change in the solute (J/mol) and  is the resultant temperature change (K). You can select among several salts and specify the number of moles added to determine the final temperature of the water in the heat or cold pack.
is the resultant temperature change (K). You can select among several salts and specify the number of moles added to determine the final temperature of the water in the heat or cold pack.
Contributed by: Alex Cam and Aaiz Hussain (January 2019)
Additional contributions by: Eitan Geva (University of Michigan)
Open content licensed under CC BY-NC-SA
Details
Reference
[1] V. B. Parker, Thermal Properties of Aqueous Uni-univalent Electrolytes, Washington DC: National Bureau of Standards, 1965. nvlpubs.nist.gov/nistpubs/Legacy/NSRDS/nbsnsrds2.pdf.
Submission from the Compute-to-Learn course at the University of Michigan.
Snapshots
Permanent Citation