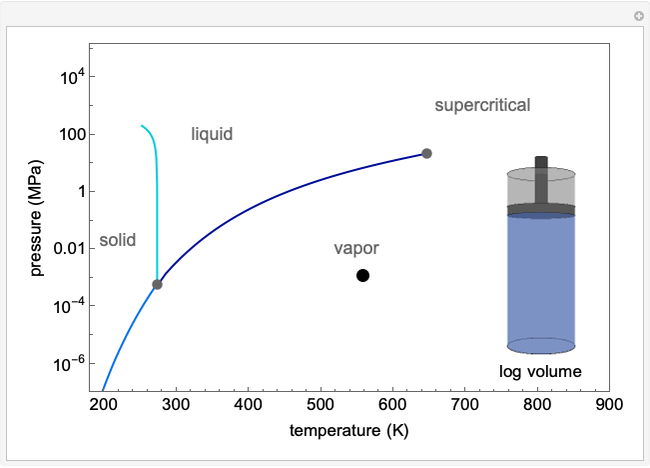
Carbon Dioxide Sublimation and Dissolution Equilibria

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
In this Demonstration, you can manipulate the number of moles of  (
( ) and the temperature
) and the temperature  (°C) to visualize both the equilibria of sublimation of solid
(°C) to visualize both the equilibria of sublimation of solid  and the dissolution of gaseous
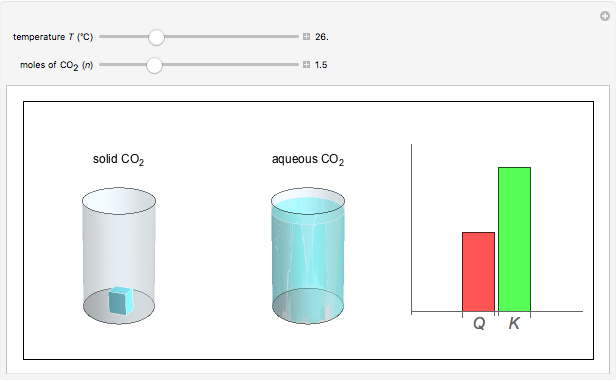
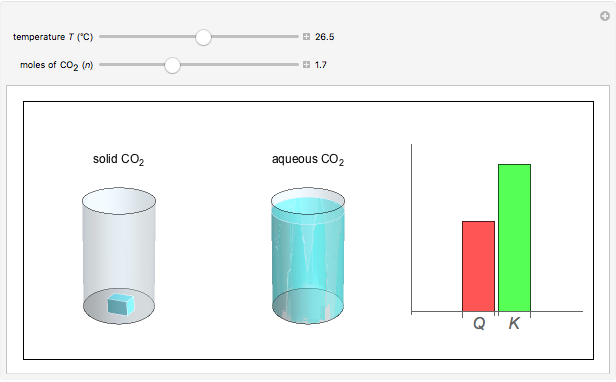
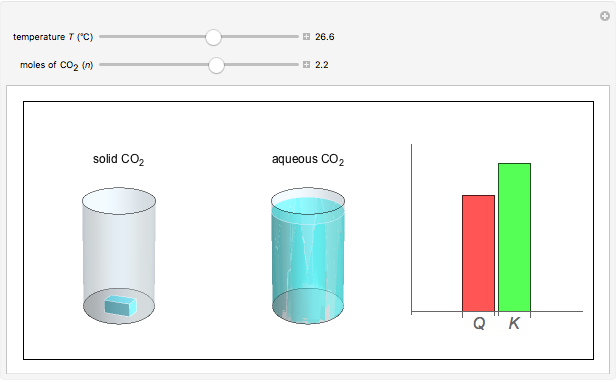
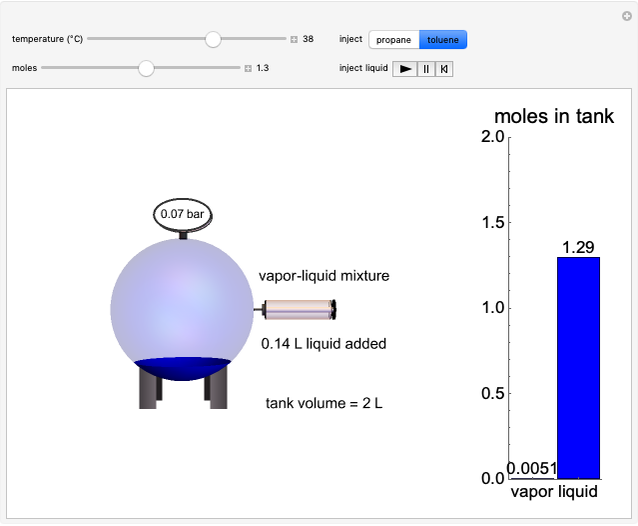
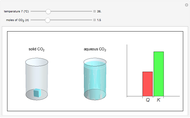
and the dissolution of gaseous  into aqueous solution. In the graphic, the left cylinder shows the sublimation of
into aqueous solution. In the graphic, the left cylinder shows the sublimation of  , beginning with solid
, beginning with solid  . The equilibrium depends on the number of moles and the temperature. In the right cylinder, the dissolution of gaseous
. The equilibrium depends on the number of moles and the temperature. In the right cylinder, the dissolution of gaseous  depends both on the Henry's law constant
depends both on the Henry's law constant  and the ideal gas law (
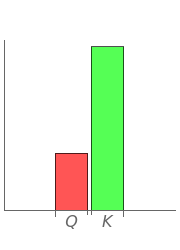
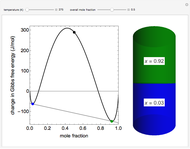
and the ideal gas law ( ). On the right is a bar graph comparing the values of
). On the right is a bar graph comparing the values of  , the reaction quotient, and
, the reaction quotient, and  , the equilibrium constant.
, the equilibrium constant.  will change as the molar concentration of carbon dioxide changes, while
will change as the molar concentration of carbon dioxide changes, while  will only change with temperature. The visual comparison of
will only change with temperature. The visual comparison of  and
and  shows the direction of the reaction. If
shows the direction of the reaction. If  is smaller than
is smaller than  , the forward reaction is favored, while if
, the forward reaction is favored, while if  is larger than
is larger than  , the reverse reaction is favored.
, the reverse reaction is favored.
Contributed by: Nathan Lwo and Kara McConaghy (March 2017)
Additional contributions by: Eitan Geva (University of Michigan)
Open content licensed under CC BY-NC-SA
Details
Reference
[1] NIST. "Carbon Dioxide." (Mar 27, 2017) webbook.nist.gov/cgi/cbook.cgi?ID=C124389&Mask=10.
Submission from the Compute-to-Learn course at the University of Michigan.
Snapshots
Permanent Citation