Thermodynamic Voltage and Efficiency of Hydrogen Fuel Cells

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
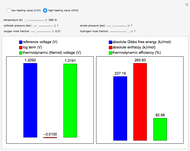
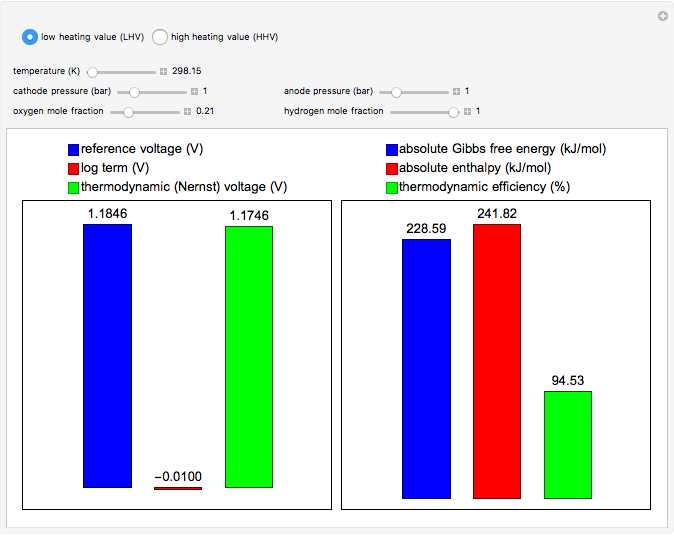
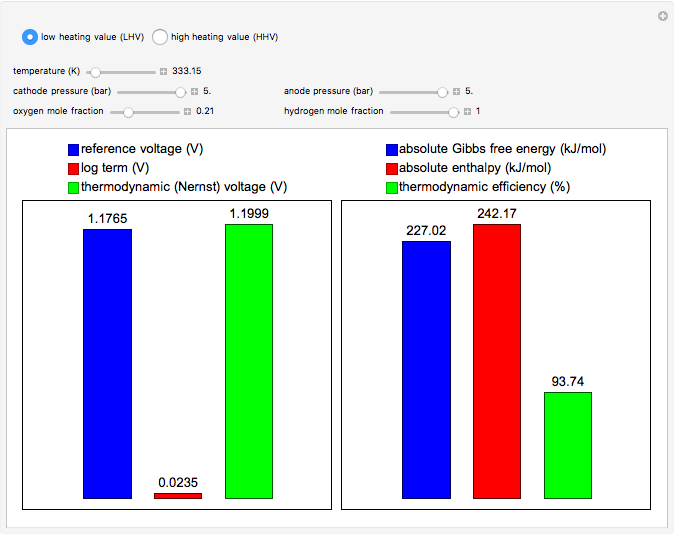
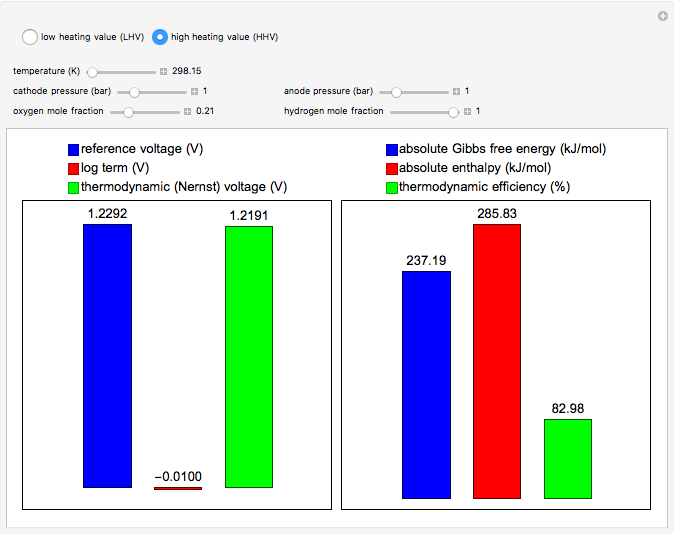
This Demonstration carries out calculations of thermodynamic voltage and efficiency for hydrogen fuel cells. It shows how temperature, pressure and composition determine the voltage and efficiency of the fuel cell. The thermodynamic voltage increases with increasing pressure and mole fraction of the reacting gases. Both thermodynamic voltage and efficiency decrease with increasing temperature. The thermodynamic efficiency changes only with temperature, since both the enthalpy and Gibbs free energy are functions of temperature alone. The calculations can be performed with lower heating value (LHV), where water is produced in the gaseous phase, and higher heating value (HHV), where water is produced in the liquid phase.
Contributed by: Mohammed S. Ismail (February 2018)
Open content licensed under CC BY-NC-SA
Snapshots
Details
$FailedPermanent Citation