Variation of Conversion with Reaction Order in a Plug Flow Reactor

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
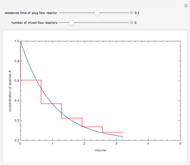
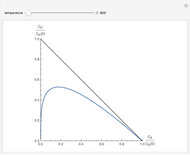
Consider an irreversible liquid phase reaction,  , taking place in a plug-flow reactor. The feed concentration is
, taking place in a plug-flow reactor. The feed concentration is  and the volumetric flow rate is
and the volumetric flow rate is  . The reaction rate law is
. The reaction rate law is  where you can vary the reaction order,
where you can vary the reaction order,  , and the reaction rate constant is held constant (e.g.,
, and the reaction rate constant is held constant (e.g.,  ). A differential mole balance gives:
). A differential mole balance gives:  where
where  is the volume of the plug flow reactor,
is the volume of the plug flow reactor,  is the conversion given by
is the conversion given by  , and
, and  is the feed flow rate of species
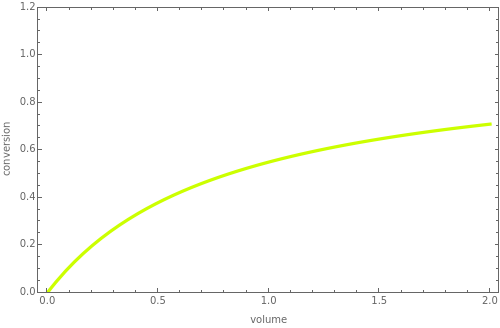
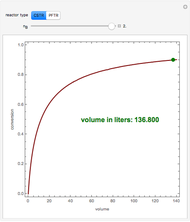
is the feed flow rate of species  . This Demonstration plots the conversion,
. This Demonstration plots the conversion,  versus the volume,
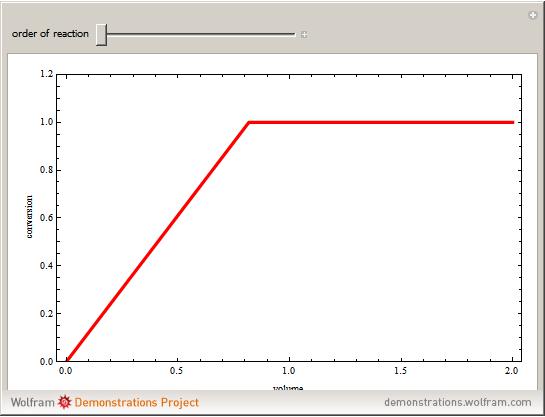
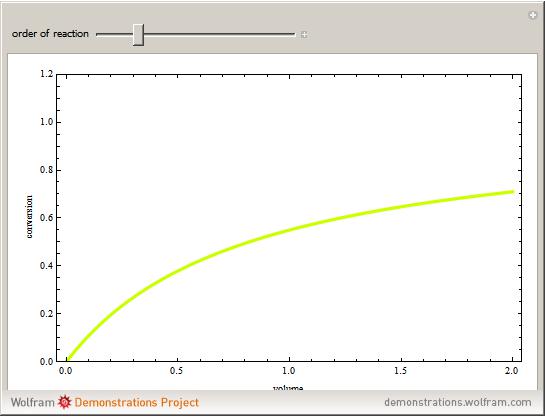
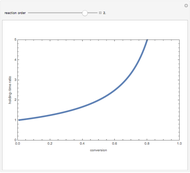
versus the volume,  , for various values of the reaction order. It is found that the conversion gets lower when the reaction order is increased.
, for various values of the reaction order. It is found that the conversion gets lower when the reaction order is increased.
Contributed by: Housam Binous (March 2011)
Open content licensed under CC BY-NC-SA
Snapshots
Details
Reference: M. B. Cutlip and M. Shacham, Problem Solving in Chemical Engineering with Numerical Methods, Upper Saddle River, NJ: Prentice Hall, 1999 (Example 8.2, "Variation of Conversion with Reaction Order in a Plug-Flow Reactor").