Distillation Column Using the Francis Formula for Flow through Weirs

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
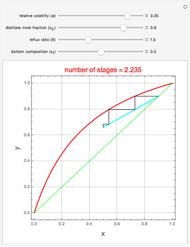
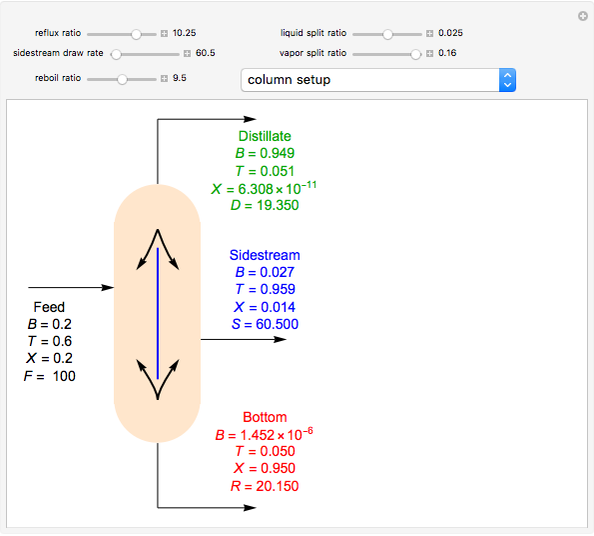
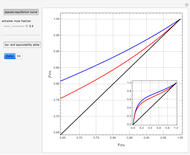
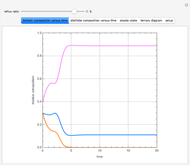
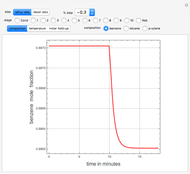
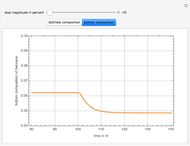
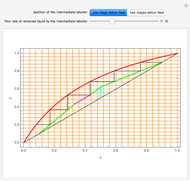
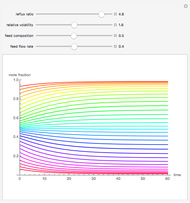
Consider a distillation column operating at atmospheric pressure with 10 stages, a partial reboiler, and a total condenser. An equimolar mixture composed of ethanol and water is to be separated by this distillation column. The feed is a saturated liquid with a flow rate equal to 10 kmol/min. Feed enters at stage 8, counting from the top. At normal operating conditions, the reflux and reboil ratios are set equal to 10 and 15, respectively. If you assume (1) a uniform tray spacing equal to 24 in and (2) an operating vapor-phase velocity equal to 80% of the value of the flooding velocity, then the column diameter can be calculated and is equal to 3.394 m. The active area is set equal to  (i.e. a fraction of the total cross-sectional area of the column).
(i.e. a fraction of the total cross-sectional area of the column).
Contributed by: Housam Binous and Ahmed Bellagi (November 2012)
Open content licensed under CC BY-NC-SA
Snapshots
Details
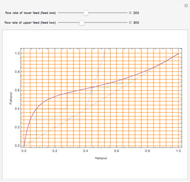
The Francis formula for flow through weirs is given by:
 ,
,
where  is the molar holdup at stage
is the molar holdup at stage  in kmols,
in kmols,  is the weir height in m,
is the weir height in m,  is the molar flow rate in kmol/min,
is the molar flow rate in kmol/min,  is the gravitational acceleration,
is the gravitational acceleration,  is the active area,
is the active area,  is the weir length (calculated from the knowledge of the active area and from pure geometrical considerations), and
is the weir length (calculated from the knowledge of the active area and from pure geometrical considerations), and  is the liquid density at stage
is the liquid density at stage  .
.
Expressions for pure component molar liquid densities and vapor and liquid enthalpies were adapted from Aspen HYSYS.
The mixture is assumed to obey modified Raoult's law, and activity coefficients are predicted using the Wilson model [1].
Reference
[1] G. M. Wilson, "Vapor-Liquid Equilibrium XI: A New Expression for the Excess Free Energy of Mixing," Journal of the American Chemical Society, 86(2), 1964 pp. 127–130.
Permanent Citation