Experiment Verifying Charles's Law

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
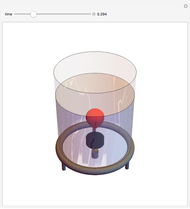
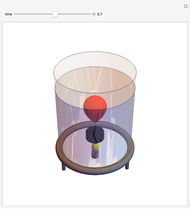
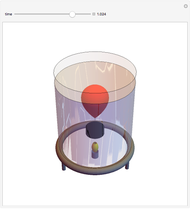
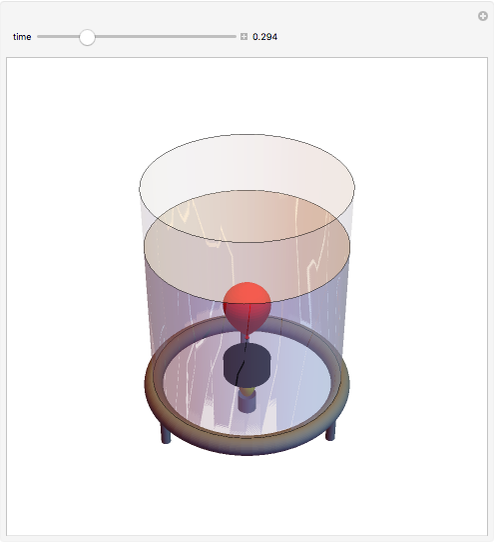
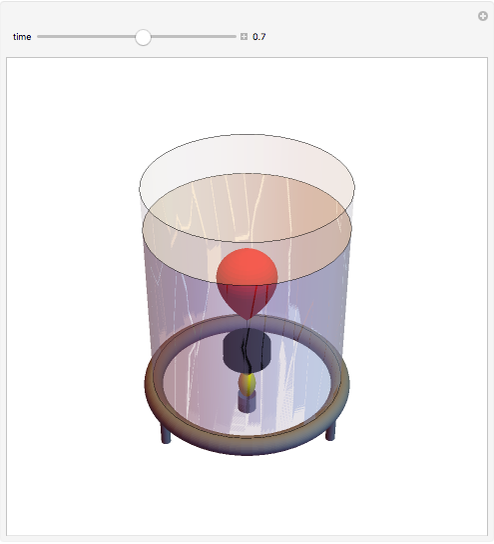
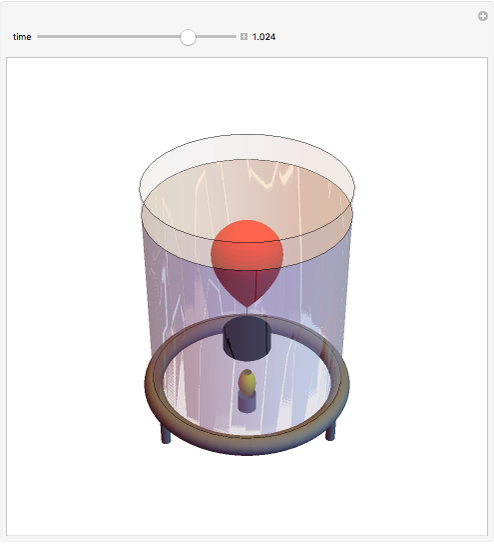
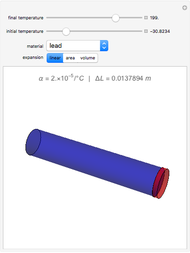
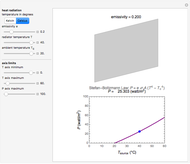
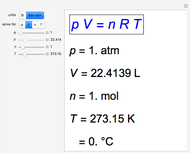
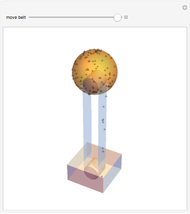
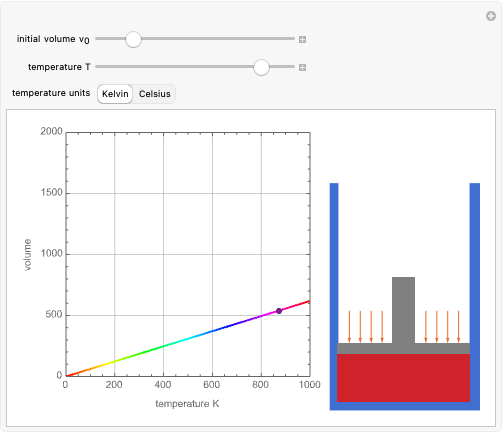
This simple experiment verifies Charles's law, that in a gas under constant pressure, the volume is proportional to the absolute temperature. A balloon is attached above a small weight at the bottom of a receptacle filled with cold water. As the receptacle is heated, the balloon inflates and its buoyancy can lift the attached weight. Cooling with ice instead of heating would instead reduce the volume of the balloon.
Contributed by: Enrique Zeleny (March 2011)
Open content licensed under CC BY-NC-SA
Snapshots
Details
detailSectionParagraphPermanent Citation
"Experiment Verifying Charles's Law"
http://demonstrations.wolfram.com/ExperimentVerifyingCharlessLaw/
Wolfram Demonstrations Project
Published: March 7 2011