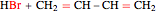Kinetic and Thermodynamic Control of Electrophilic Addition Reactions

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
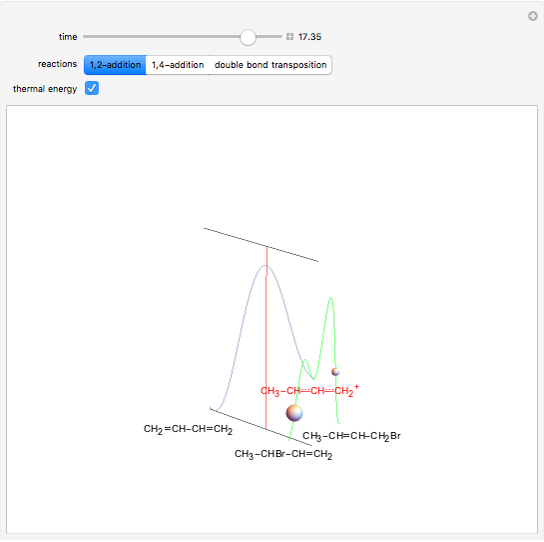
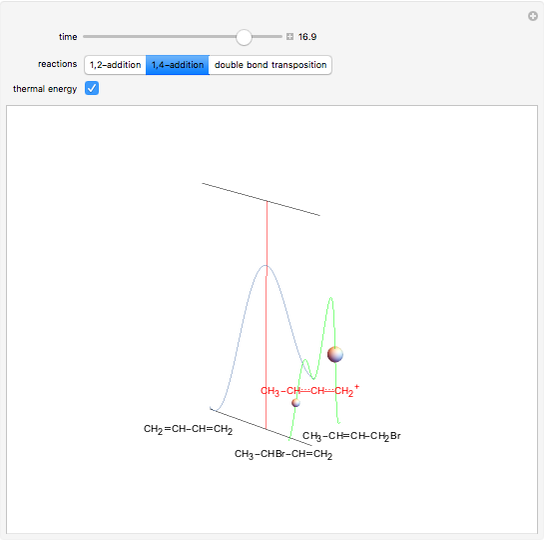
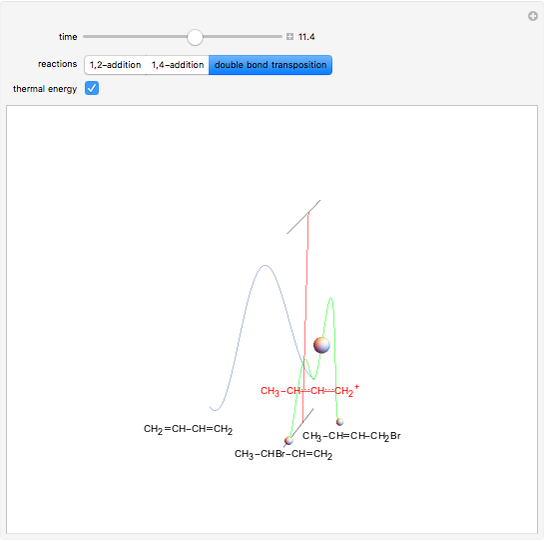
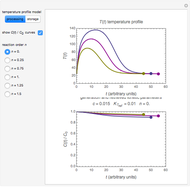
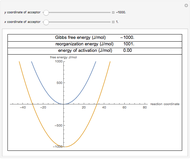
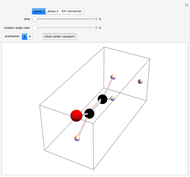
This Demonstration shows the effect of thermodynamics and kinetics on electrophilic addition reactions producing dienes. The following reaction is considered:
[more]
Contributed by: D. Meliga and S. Z. Lavagnino (March 2018)
Open content licensed under CC BY-NC-SA
Snapshots
Details
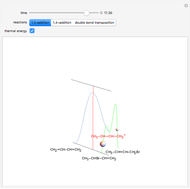
Snapshot 1: the lower thermal energy promotes the predominance of the 1,2-addition compared to the 1,4-addition
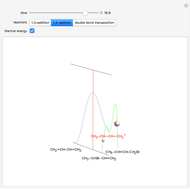
Snapshot 2: the higher thermal energy promotes the predominance of the 1,4-addition compared to the 1,2-addition
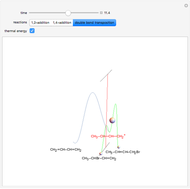
Snapshot 3: increasing the temperature causes the transfer of the double bond
References
[1] R. T. Morrison and R. N. Boyd, Organic Chemistry, 3rd ed., Boston: Allyn and Bacon, 1973.
[2] Chemistry LibreTexts. "16.10: Electrophilic Addition: 1,2- versus 1,4-Addition." (Feb 16, 2018) chem.libretexts.org/?title=Textbook_Maps/Organic_Chemistry_Textbook_Maps/Map:_Organic_Chemistry_(Smith)/Chapter_16:_Conjugation,_Resonance,_%26_Dienes/16.10:_Electrophilic_Addition:_1,2-_Versus_1,4-Addition.
Permanent Citation