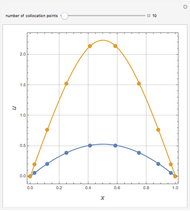
Reaction Factor for Second-Order Gas-Liquid Reactions

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
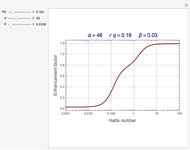
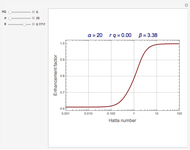
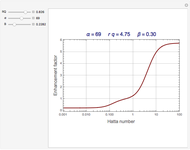
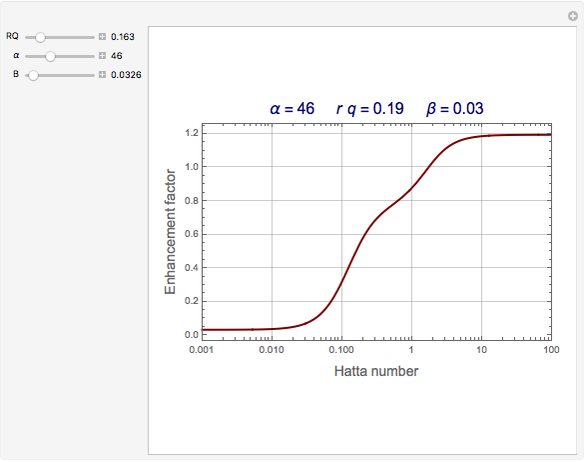
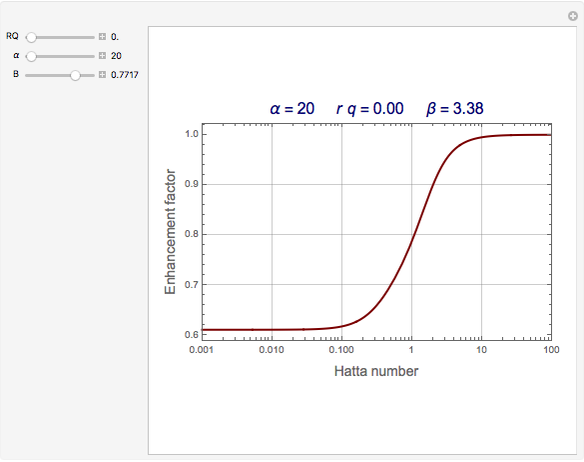
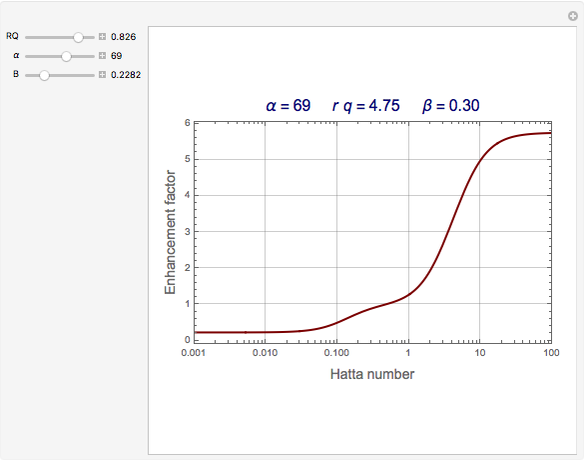
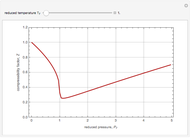
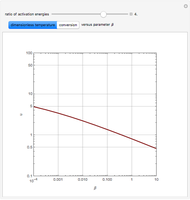
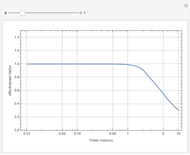
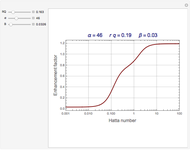
The Demonstration plots the reaction factor for a second-order gas-liquid reaction versus the Hatta number, 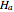 , for various values of the parameters,
, for various values of the parameters,  ,
, 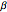 , and
, and 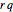 defined in Details. Two new parameters are introduced and defined by
defined in Details. Two new parameters are introduced and defined by 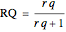 and
and 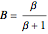 ; both vary from
; both vary from  to
to 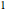 when
when 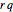 and
and 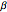 vary from 0 to
vary from 0 to 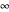 .
.
Contributed by: Housam Binousand Abdullah A. Shaikh (April 2011)
(King Fahd University of Petroleum & Minerals)
Open content licensed under CC BY-NC-SA
Snapshots
Details
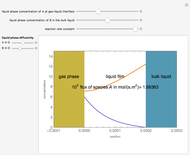
Consider a second-order reaction between dissolved gaseous species 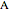 and a nonvolatile liquid species
and a nonvolatile liquid species 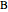 . The reaction factor for this second-order gas-liquid reaction is given by:
. The reaction factor for this second-order gas-liquid reaction is given by:
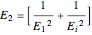 , where
, where 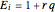 and
and 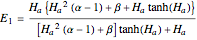 .
.
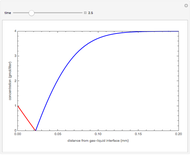
The Hatta number, 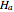 , is a dimensionless parameter defined as the ratio of the rate of chemical absorption to the rate of physical absorption for species
, is a dimensionless parameter defined as the ratio of the rate of chemical absorption to the rate of physical absorption for species 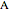 . Here
. Here 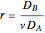 (where
(where  is a stoichiometric coefficient for species
is a stoichiometric coefficient for species 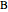 ),
), 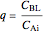 gives ratios of diffusivities and concentrations, and
gives ratios of diffusivities and concentrations, and 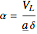 and
and 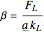 are two dimensionless parameters. All parameters are defined in [1].
are two dimensionless parameters. All parameters are defined in [1].
Reference
[1] A. A. Shaikh and S. Zarook, "An Explicit Reaction Factor for General Second-Order Gas-Liquid Reactions," Journal of Chemical Engineering of Japan, 28(3), 1995 pp. 346–348.
Permanent Citation