The Law of Mass Action

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
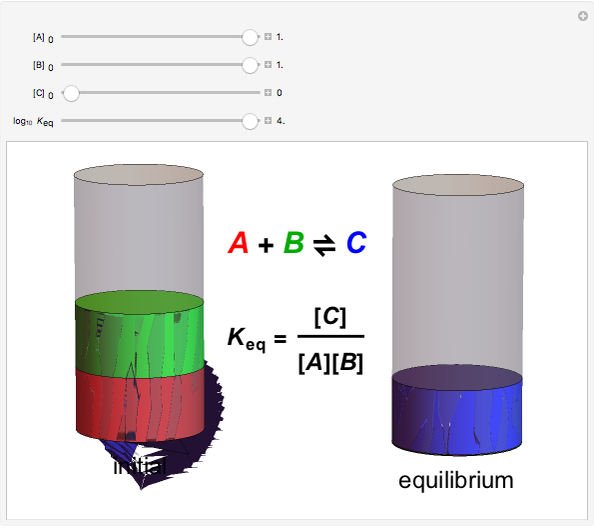
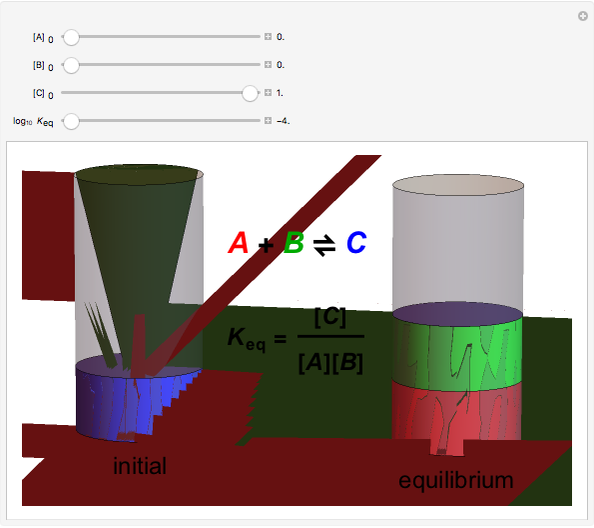
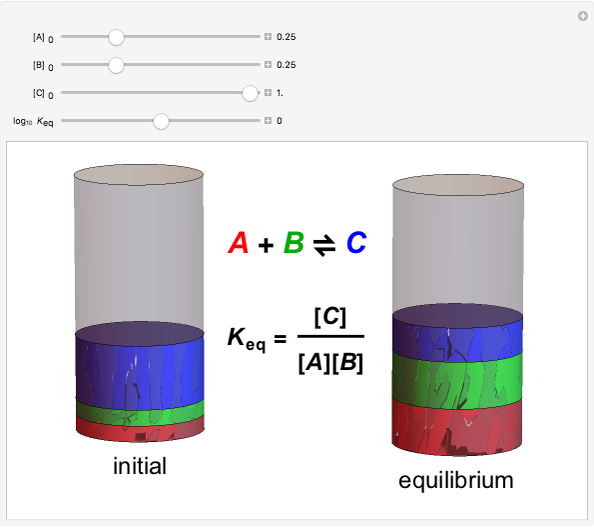
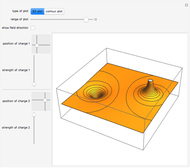
The law of mass action is one example of Le Chatelier's principle, that the equilibrium in a chemical system responds to a change in concentration, temperature, or pressure by shifting in the direction which partially counteracts the imposed perturbation. Thus if the reaction considered were exothermic, such that heat could formally be regarded as one of the products, an increase in temperature would shift the equilibrium to the left. If A, B and C were gases, an increase in total pressure would shift the equilibrium to the right.
Contributed by: S. M. Blinder (March 2011)
Open content licensed under CC BY-NC-SA
Snapshots
Details
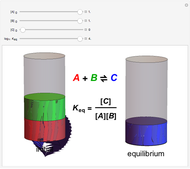
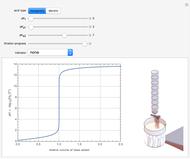
Snapshot 1: when 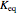 is large, the reactants
is large, the reactants 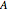 and
and 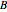 are almost completely converted into product
are almost completely converted into product 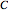
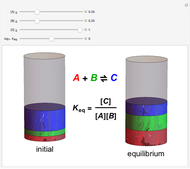
Snapshot 2: conversely, for small 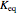 , pure
, pure 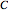 will almost entirely convert to
will almost entirely convert to 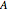 and
and 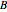
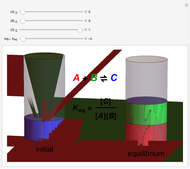
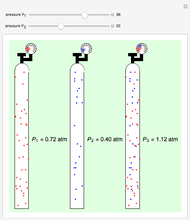
Snapshot 3: adding more 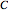 to the reaction mixture will shift the equilibrium toward higher concentrations of
to the reaction mixture will shift the equilibrium toward higher concentrations of 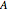 and
and 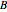
Permanent Citation