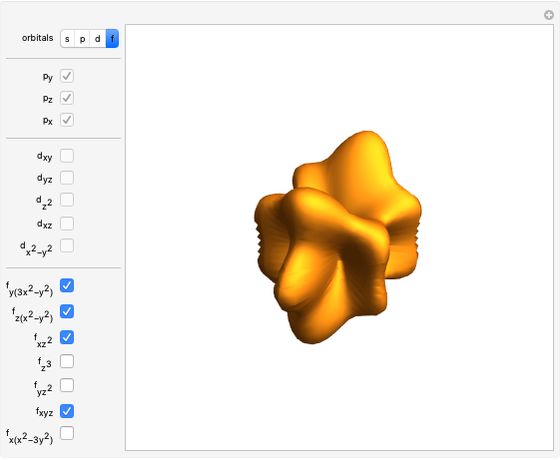
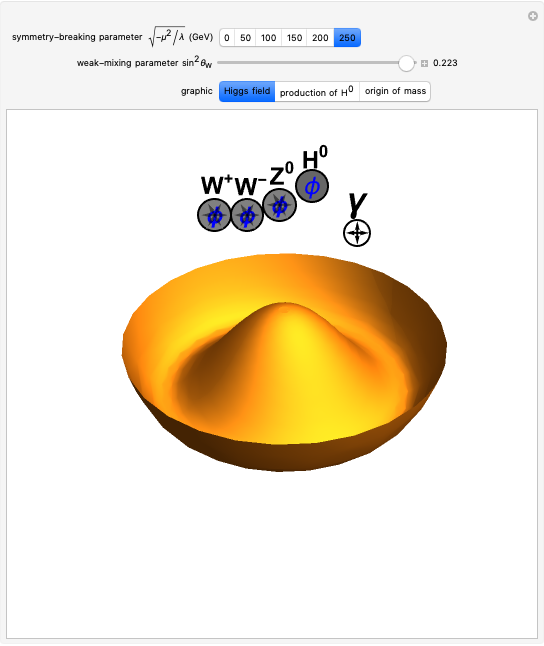
Unsöld's Theorem
Initializing live version

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
Contributed by: S. M. Blinder (March 2011)
Open content licensed under CC BY-NC-SA
Snapshots
Details
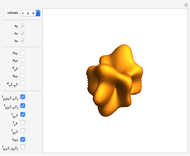
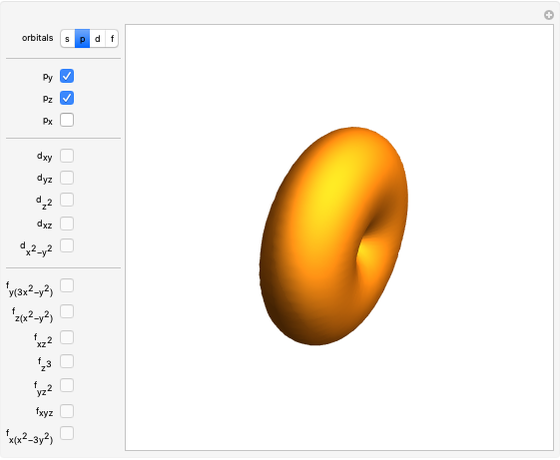
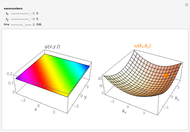
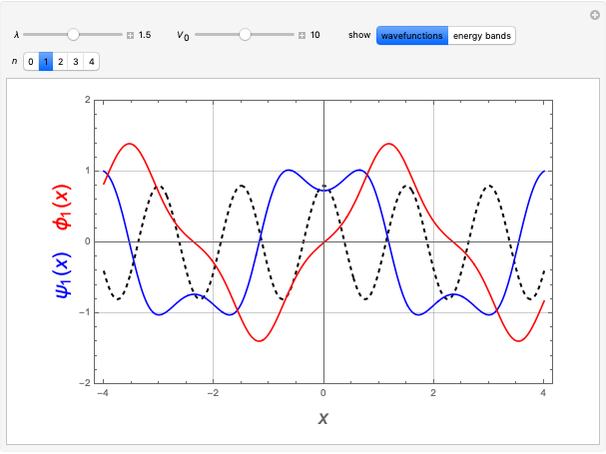
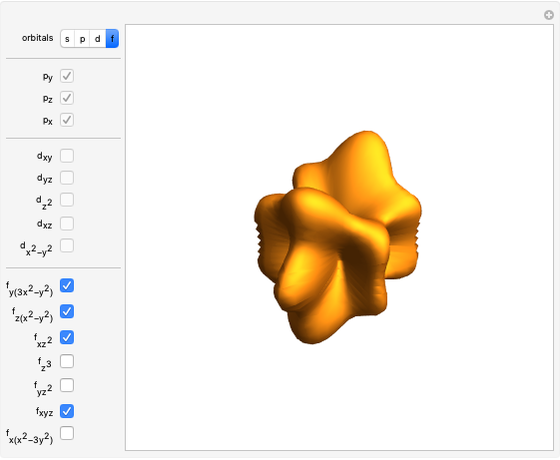
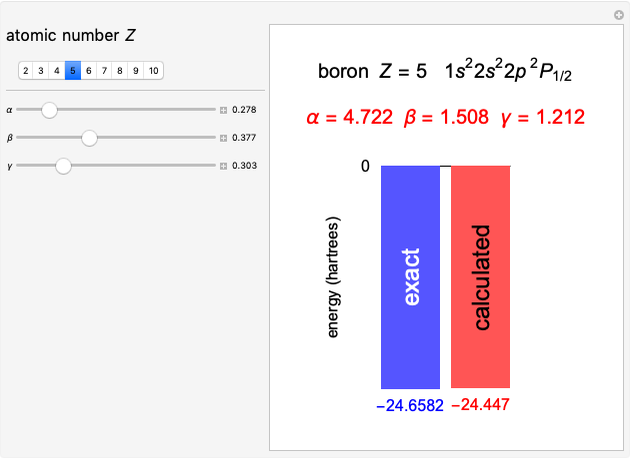
Snapshot 1: a 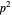 configuration, showing a
configuration, showing a 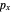 orbital hole
orbital hole
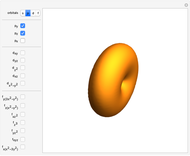
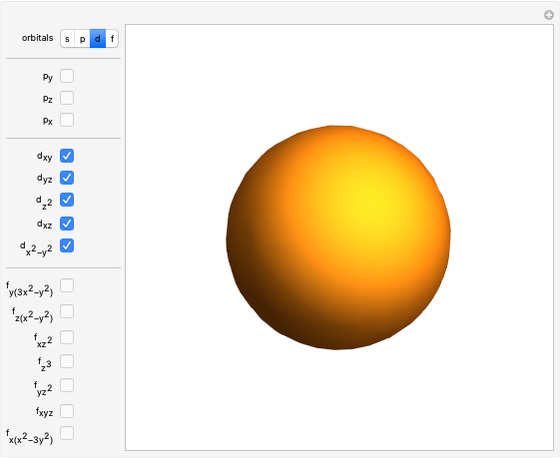
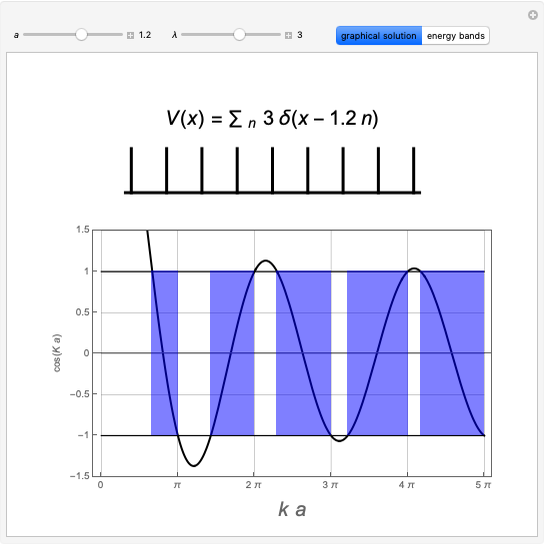
Snapshot 2: spherically symmetrical 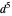 or
or 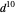 configuration
configuration
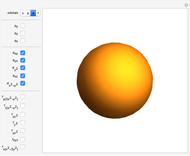
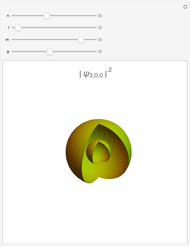
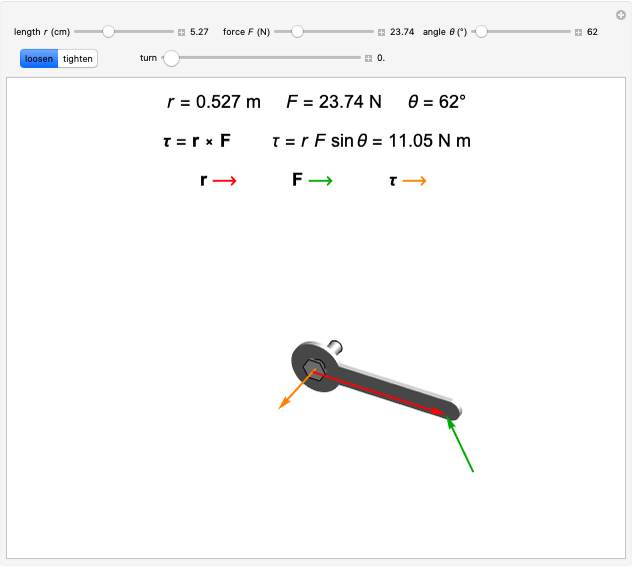
Snapshot 3: 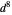 configuration, which accounts for square-planar complexes of Ni, Pd and Pt
configuration, which accounts for square-planar complexes of Ni, Pd and Pt
Permanent Citation