Absorption and Emission of Radiation by Atoms

Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
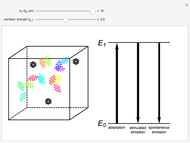
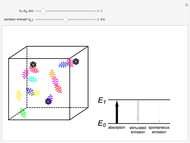
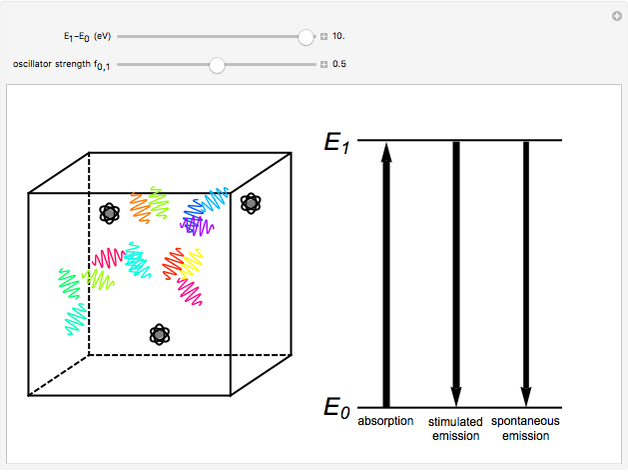
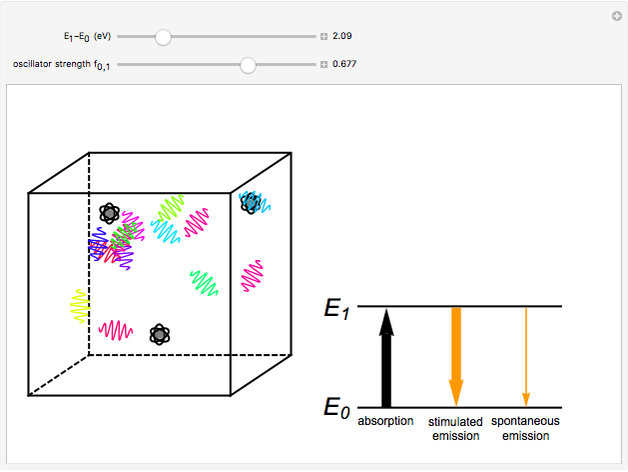
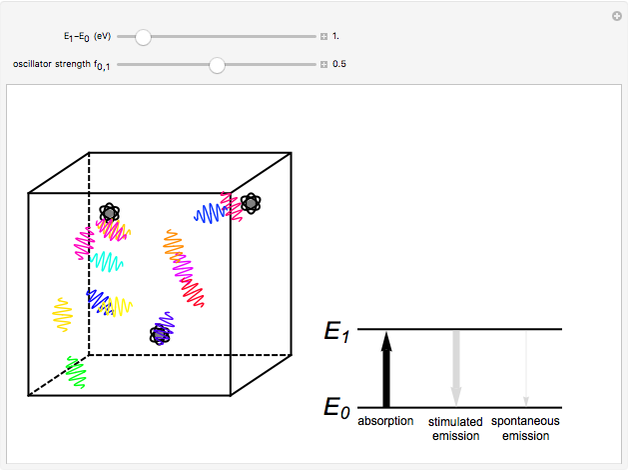
The quantum theory of radiation pictures atoms with quantized energy levels interacting with an assembly of photons of varying frequencies  . This can be represented by the cubic box on the left side of the graphic. When the energy difference between two atomic states fulfills a resonance condition
. This can be represented by the cubic box on the left side of the graphic. When the energy difference between two atomic states fulfills a resonance condition  , three possible transition processes can occur: absorption, stimulated emission, and spontaneous emission. In the electric-dipole approximation, the transition rates for absorption and stimulated emission are given by
, three possible transition processes can occur: absorption, stimulated emission, and spontaneous emission. In the electric-dipole approximation, the transition rates for absorption and stimulated emission are given by  , where
, where  is the electric dipole operator and
is the electric dipole operator and  is the spectral density of the radiation field at frequency
is the spectral density of the radiation field at frequency  . The corresponding rate of spontaneous emission is
. The corresponding rate of spontaneous emission is 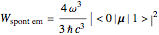 . All three radiative processes depend on the matrix element
. All three radiative processes depend on the matrix element  and therefore obey the same selection rules. A convenient measure of the intensity of a transition is the oscillator strength
and therefore obey the same selection rules. A convenient measure of the intensity of a transition is the oscillator strength  , which generally lies in the range from 0 to 1. The dependence on
, which generally lies in the range from 0 to 1. The dependence on  makes spontaneous emission significant only for higher-energy transitions—in practice, only for optical frequencies and higher. This Demonstration considers the transition-energy range of 0.1 to 10 eV, corresponding to radiation wavelengths of 120 to 12000 nm, which includes the visible region 380–750 nm, as well as parts of the ultraviolet and infrared.
makes spontaneous emission significant only for higher-energy transitions—in practice, only for optical frequencies and higher. This Demonstration considers the transition-energy range of 0.1 to 10 eV, corresponding to radiation wavelengths of 120 to 12000 nm, which includes the visible region 380–750 nm, as well as parts of the ultraviolet and infrared.
Contributed by: S. M. Blinder (March 2011)
Open content licensed under CC BY-NC-SA
Snapshots
Details
Snapshot 1: a transition in the ultraviolet region
Snapshot 2: the sodium  line at 589 nm, with an oscillator strength of 0.677
line at 589 nm, with an oscillator strength of 0.677
Snapshot 3: a transition in the infrared region; note that the spontaneous emission becomes very weak
Reference: S. M. Blinder, Introduction to Quantum Mechanics, Amsterdam: Elsevier, 2004 pp. 68–71.
Permanent Citation